Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Orexin receptor-1 expression in edult rodent neurogenic regions: evidence from the subgranular zone and the median eminence
1
Department of Histology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Corresponding author
Artur Pałasz
Zakład Histologii, Wydział Nauk Medycznych w Katowicach ŚUM, ul. Medyków 18, 40-752 Katowice
Zakład Histologii, Wydział Nauk Medycznych w Katowicach ŚUM, ul. Medyków 18, 40-752 Katowice
Ann. Acad. Med. Siles. 2025;79:308-315
KEYWORDS
TOPICS
ABSTRACT
Introduction:
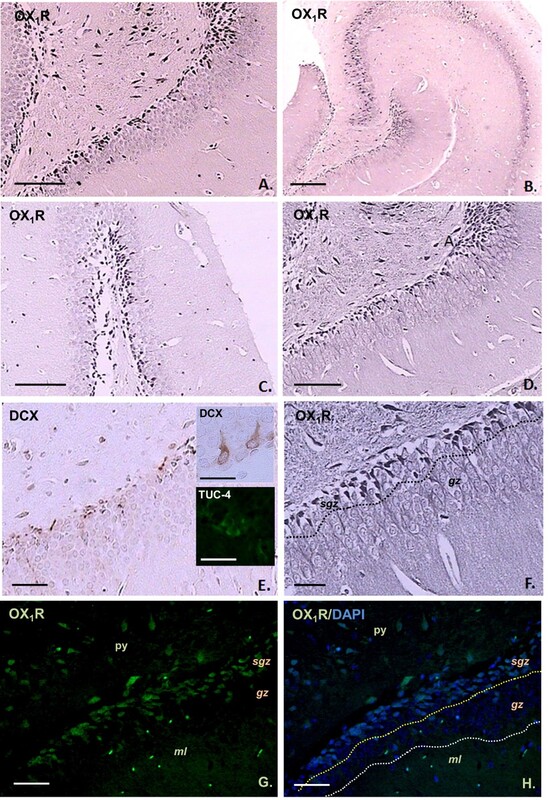
Orexin signaling plays a vital role in regulating autonomic and cognitive functions, including sleep-wake cycles, feeding, and memory. Orexin receptor-1 (OX1R), a key component of this system, may also influence adult neurogenesis. This study examined OX1R expression in both classical (hippocampal) and non-classical (hypothalamic) neurogenic regions of the adult rodent brain.
Material and Methods:
Adult rodent brains were fixed, paraffin-embedded, and sectioned coronally. Immunohistochemistry and immunofluorescence were performed using antibodies against OX1R, DCX, and TUC-4, followed by fluorophore- or diaminobenzidine-based detection. Negative controls were included to ensure specificity.
Results:
OX1R-positive cells were localized primarily to the subgranular zone (SGZ) of the dentate gyrus and β2 tanycytes of the median eminence showed uniform OX1R expression in both somata and vascular-directed processes. Morphological variation was observed between species, with diverse perikaryon shapes in mice and predominantly elongated, multipolar forms in rats.
Conclusions:
This study revealed, for the first time, region-specific OX1R expression in the SGZ in β2 tanycytes of the median eminence. These findings suggest a potential role for orexin signaling in adult neurogenesis.
Orexin signaling plays a vital role in regulating autonomic and cognitive functions, including sleep-wake cycles, feeding, and memory. Orexin receptor-1 (OX1R), a key component of this system, may also influence adult neurogenesis. This study examined OX1R expression in both classical (hippocampal) and non-classical (hypothalamic) neurogenic regions of the adult rodent brain.
Material and Methods:
Adult rodent brains were fixed, paraffin-embedded, and sectioned coronally. Immunohistochemistry and immunofluorescence were performed using antibodies against OX1R, DCX, and TUC-4, followed by fluorophore- or diaminobenzidine-based detection. Negative controls were included to ensure specificity.
Results:
OX1R-positive cells were localized primarily to the subgranular zone (SGZ) of the dentate gyrus and β2 tanycytes of the median eminence showed uniform OX1R expression in both somata and vascular-directed processes. Morphological variation was observed between species, with diverse perikaryon shapes in mice and predominantly elongated, multipolar forms in rats.
Conclusions:
This study revealed, for the first time, region-specific OX1R expression in the SGZ in β2 tanycytes of the median eminence. These findings suggest a potential role for orexin signaling in adult neurogenesis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES (56)
1.
Gonçalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 2016; 167(4): 897–914, doi: 10.1016/j.cell.2016.10.021.
2.
Rolando C., Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr. Top. Dev. Biol. 2014; 107: 183–206, doi: 10.1016/B978-0-12-416022-4.00007-X.
3.
Kokoeva M.V., Yin H., Flier J.S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 2005; 310(5748): 679–683, doi: 10.1126/science.1115360.
4.
Pierce A.A., Xu A.W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. 2010; 30(2): 723–730, doi: 10.1523/JNEUROSCI.2479-09.2010.
5.
Goodman T., Hajihosseini M.K. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci. 2015; 9: 387, doi: 10.3389/fnins.2015.00387.
6.
Mathew T.C. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat. Histol. Embryol. 2008; 37(1): 9–18, doi: 10.1111/j.1439-0264.2007.00786.x.
7.
Prevot V., Dehouck B., Sharif A., Ciofi P., Giacobini P., Clasadonte J. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 2018; 39(3): 333–368, doi: 10.1210/er.2017-00235.
8.
Mullier A., Bouret S.G., Prevot V., Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 2010; 518(7): 943–962, doi: 10.1002/cne.22273.
9.
Wei L.C., Shi M., Chen L.W., Cao R., Zhang P., Chan Y.S. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res. Dev. Brain Res. 2002; 139(1): 9–17, doi: 10.1016/s0165-3806(02)00509-6.
10.
Lee D.A., Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int. J. Dev. Neurosci. 2012; 30(8): 615–621, doi: 10.1016/j.ijdevneu.2012.07.003.
11.
Bolborea M., Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013; 36(2): 91–100, doi: 10.1016/j.tins.2012.12.008.
12.
Saaltink D.J., Håvik B., Verissimo C.S., Lucassen P.J., Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell populations: implications for neurogenesis. J. Comp. Neurol. 2012; 520(13): 2805–2823, doi: 10.1002/cne.23144.
13.
Bonnavion P., de Lecea L. Hypocretins in the control of sleep and wakefulness. Curr. Neurol. Neurosci. Rep. 2010; 10(3): 174–179, doi: 10.1007/s11910-010-0101-y.
14.
Nattie E., Li A. Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J. Appl. Physiol. (1985) 2010; 108(5): 1417–1424, doi: 10.1152/japplphysiol.01261.2009.
15.
Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G. et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998; 18(23): 9996–10015, doi: 10.1523/JNEUROSCI.18-23-09996.1998.
16.
Torrealba F., Yanagisawa M., Saper C.B. Colocalization of orexin A and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 2003; 119(4): 1033–1044, doi: 10.1016/s0306-4522(03)00238-0.
17.
Chou T.C., Lee C.E., Lu J., Elmquist J.K., Hara J., Willie J.T. et al. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci. 2001; 21(19): RC168, doi: 10.1523/JNEUROSCI.21-19-j0003.2001.
18.
Nambu T., Sakurai T., Mizukami K., Hosoya Y., Yanagisawa M., Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999; 827(1–2): 243–260, doi: 10.1016/s0006-8993(99)01336-0.
19.
van den Pol A.N. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 1999; 19(8): 3171–3182, doi: 10.1523/JNEUROSCI.19-08-03171.1999.
20.
Tsujino N., Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol. Rev. 2009; 61(2): 162–176, doi: 10.1124/pr.109.001321.
21.
Marcus J.N., Aschkenasi C.J., Lee C.E., Chemelli R.M., Saper C.B., Yanagisawa M. et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001; 435(1): 6–25, doi: 10.1002/cne.1190.
22.
Trivedi P., Yu H., MacNeil D.J., Van der Ploeg L.H., Guan X.M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998; 438(1–2): 71–75, doi: 10.1016/s0014-5793(98)01266-6.
23.
Arias-Carrion O., Ortega-Robles E., de Celis-Alonso B., Palasz A., Mendez-Rojas M.A., Salas-Pacheco J. et al. Depletion of hypocretin/orexin neurons increases cell proliferation in the adult subventricular zone. CNS Neurol. Disord. Drug Targets 2018; 17(2): 106–112, doi: 10.2174/1871527317666180314115623.
24.
Barson J.R., Leibowitz S.F. Orexin/hypocretin system: role in food and drug overconsumption. Int. Rev. Neurobiol. 2017; 136: 199–237, doi: 10.1016/bs.irn.2017.06.006.
25.
Nevárez N., de Lecea L. Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Res. 2018; 7: F1000 Faculty Rev-1421, doi: 10.12688/f1000research.15097.1.
26.
Sargin D. The role of the orexin system in stress response. Neuropharmacology 2019; 154: 68–78, doi: 10.1016/j.neuropharm.2018.09.034.
27.
Haghparast A., Fatahi Z., Arezoomandan R., Karimi S., Taslimi Z., Zarrabian S. Functional roles of orexin/hypocretin receptors in reward circuit. Prog. Brain Res. 2017; 235: 139–154, doi: 10.1016/bs.pbr.2017.08.005.
28.
Wang C., Wang Q., Ji B., Pan Y., Xu C., Cheng B. et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front. Mol. Neurosci. 2018; 11: 220, doi: 10.3389/fnmol.2018.00220.
29.
Chien Y.L., Liu C.M., Shan J.C., Lee H.J., Hsieh M.H., Hwu H.G. et al. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology 2015; 53: 1–9, doi: 10.1016/j.psyneuen.2014.12.012.
30.
Lu X.Y., Bagnol D., Burke S., Akil H., Watson S.J. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm. Behav. 2000; 37(4): 335–344, doi: 10.1006/hbeh.2000.1584.
31.
Alizamini M.M., Kavianpour M., Karimi-Haghighi S., Fatahi Z., Haghparast A. Intra-hippocampal administration of orexin receptor antagonists dose-dependently attenuates reinstatement of morphine seeking behavior in extinguished rats. Peptides 2018; 110: 40–46, doi: 10.1016/j.peptides.2018.10.011.
32.
Mavanji V., Butterick T.A., Duffy C.M., Nixon J.P., Billington C.J., Kotz C.M. Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol. Learn. Mem. 2017; 146: 21–30, doi: 10.1016/j.nlm.2017.10.014.
33.
Sadeghi B., Ezzatpanah S., Haghparast A. Effects of dorsal hippocampal orexin-2 receptor antagonism on the acquisition, expression, and extinction of morphine-induced place preference in rats. Psychopharmacology 2016; 233(12): 2329–2341, doi: 10.1007/s00213-016-4280-3.
34.
Akbari E., Naghdi N., Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res. 2006; 173(1): 47–52, doi: 10.1016/j.bbr.2006.05.028.
35.
Bahramzadeh Zoeram S., Elahdadi Salmani M., Lashkarbolouki T., Goudarzi I. Hippocampal orexin receptor blocking prevented the stress-induced social learning and memory deficits. Neurobiol. Learn. Mem. 2019; 157: 12–23, doi: 10.1016/j.nlm.2018.11.009.
36.
Edalat P., Kavianpour M., Zarrabian S., Haghparast A. Role of orexin-1 and orexin-2 receptors in the CA1 region of hippocampus in the forced swim stress- and food deprivation-induced reinstatement of morphine seeking behaviors in rats. Brain Res. Bull. 2018; 142: 25–32, doi: 10.1016/j.brainresbull.2018.06.016.
37.
Pourreza P., Babapour V., Haghparast A. Role of dorsal hippocampal orexin-1 receptors in modulation of antinociception induced by chemical stimulation of the lateral hypothalamus. Physiol. Behav. 2018; 185: 79–86, doi: 10.1016/j.physbeh.2017.12.036.
38.
Brojeni M.S., Rashvand M., Haghparast A. Role of orexin receptors within the dentate gyrus of the hippocampus in antinociception induced by chemical stimulation of the lateral hypothalamus in the tail-flick test as a model of acute pain in rats. Physiol. Behav. 2019; 209: 112595, doi: 10.1016/j.physbeh.2019.112595.
39.
Chieffi S., Carotenuto M., Monda V., Valenzano A., Villano I., Precenzano F. et al. Orexin system: the key for a healthy life. Front. Physiol. 2017; 8: 357, doi: 10.3389/fphys.2017.00357.
40.
Li S.B., Jones J.R., de Lecea L. Hypocretins, neural systems, physiology, and psychiatric disorders. Curr. Psychiatry Rep. 2016; 18(1): 7, doi: 10.1007/s11920-015-0639-0.
41.
Wayner M.J., Armstrong D.L., Phelix C.F., Oomura Y. Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 2004; 25(6): 991–996, doi: 10.1016/j.peptides.2004.03.018.
42.
Yang L., Zou B., Xiong X., Pascual C., Xie J., Malik A. et al. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J. Neurosci. 2013; 33(12): 5275–5284, doi: 10.1523/JNEUROSCI.3200-12.2013.
43.
Ito N., Yabe T., Gamo Y., Nagai T., Oikawa T., Yamada H. et al. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience 2008; 157(4): 720–732, doi: 10.1016/j.neuroscience.2008.09.042.
44.
Arendt D.H., Ronan P.J., Oliver K.D., Callahan L.B., Summers T.R., Summers C.H. Depressive behavior and activation of the orexin/hypocretin system. Behav. Neurosci. 2013; 127(1): 86–94, doi: 10.1037/a0031442.
45.
Zhao X., Zhang R.x., Tang S., Ren Y.y., Yang W.x., Liu X.m. et al. Orex-in-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides 2014; 54: 140–147, doi: 10.1016/j.peptides.2013.11.019.
46.
Samokhina E., Samokhin A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int. J. Neurosci. 2018; 128(11): 1086–1096, doi: 10.1080/00207454.2018.1481064.
47.
Sousa-Ferreira L., Álvaro A.R., Aveleira C., Santana M., Brandão I., Kügler S. et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and orexin-A and differentiate to functional neurons. PLoS One 2011; 6(5): e19745, doi: 10.1371/journal.pone.0019745.
48.
Recabal A., Caprile T., García-Robles M.L.A. Hypothalamic neurogenesis as an adaptive metabolic mechanism. Front. Neurosci. 2017; 11: 190, doi: 10.3389/fnins.2017.00190.
49.
Rojczyk-Gołębiewska E., Pałasz A., Wiaderkiewicz R. Hypothalamic subependymal niche: a novel site of the adult neurogenesis. Cell. Mol. Neurobiol. 2014; 34(5): 631–642, doi: 10.1007/s10571-014-0058-5.
50.
Batailler M., Droguerre M., Baroncini M., Fontaine C., Prevot V., Migaud M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: a comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014; 522(8): 1966–1985, doi: 10.1002/cne.23514.
51.
Ebling F.J. Hypothalamic control of seasonal changes in food intake and body weight. Front. Neuroendocrinol. 2015; 37: 97–107, doi: 10.1016/j.yfrne.2014.10.003.
52.
Samms R.J., Lewis J.E., Lory A., Fowler M.J., Cooper S., Warner A. et al. Antibody-mediated inhibition of the FGFR1c isoform induces a catabolic lean state in Siberian hamsters. Curr. Biol. 2015; 25(22): 2997–3003, doi: 10.1016/j.cub.2015.10.010.
53.
Rizzoti K., Lovell-Badge R. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol. Cell. Endocrinol. 2017; 445: 7–13, doi: 10.1016/j.mce.2016.08.020.
54.
Lee D.A., Bedont J.L., Pak T., Wang H., Song J., Miranda-Angulo A. et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012; 15(5): 700–702, doi: 10.1038/nn.3079.
55.
Lee D.A., Yoo S., Pak T., Salvatierra J., Velarde E., Aja S. et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front. Neurosci. 2014; 8: 157, doi: 10.3389/fnins.2014.00157.
56.
Duque A., Spector R. A balanced evaluation of the evidence for adult neurogenesis in humans: implication for neuropsychiatric disorders. Brain Struct. Funct. 2019; 224(7): 2281–2295, doi: 10.1007/s00429-019-01917-6.
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.