Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Should we exclude hemato-oncological patients from obesity treatment with semaglutide? – A case report
1
Students’ Scientific Club, Department of Internal Medicine and Oncological Chemotherapy,
Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
2
Department of Internal Medicine and Oncological Chemotherapy, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Corresponding author
Julia Dobrowolska
Studenckie Koło Naukowe, Katedra i Klinika Chorób Wewnętrznych i Chemioterapii Onkologicznej, ul. Reymonta 8, 40-027 Katowice
Studenckie Koło Naukowe, Katedra i Klinika Chorób Wewnętrznych i Chemioterapii Onkologicznej, ul. Reymonta 8, 40-027 Katowice
Ann. Acad. Med. Siles. 2025;79:316-322
KEYWORDS
TOPICS
ABSTRACT
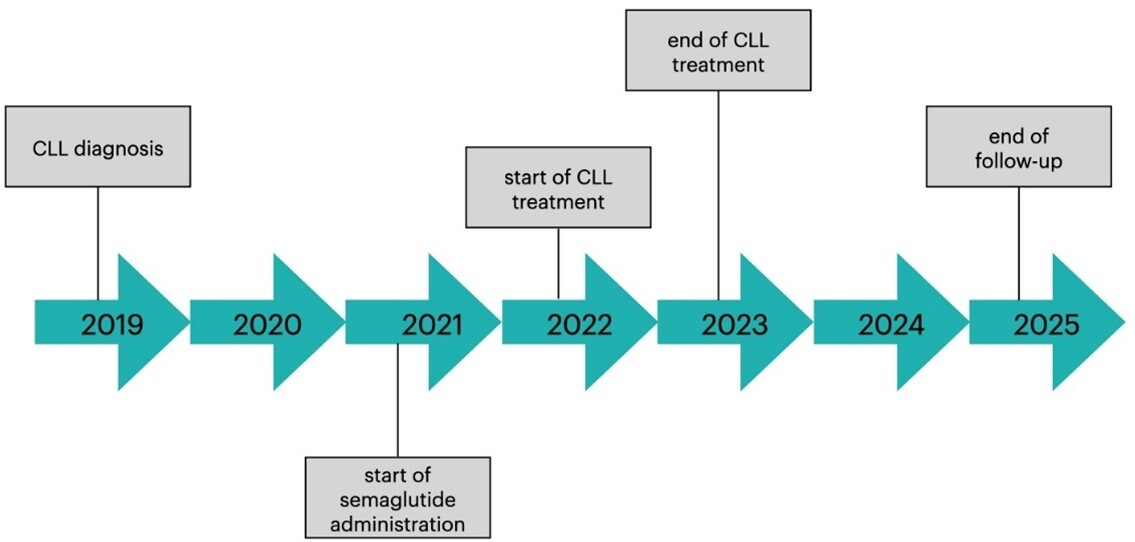
Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), is now widely used in the treatment of diabetes and obesity. However, there are still insufficient safety data for the use of GLP-1RAs in oncological and hemato-oncological patients, as they have not been included in clinical trials. The potential prooncogenic activity of GLP-1RAs in patients with thyroid cancer has been reported, raising concerns about the safety of semaglutide in oncological and hemato-oncological patients. We present a case of a 57-year-old man, who suffered from class III obesity (BMI: 40.4 kg/m2), type 2 diabetes, and chronic lymphocytic leukemia (CLL; RAI stage I and Binet A stage). The patient started therapy with semaglutide to manage obesity and diabetes; he had already begun systemic therapy for CLL with obinutuzumab and venetoclax, which was continued after its complete remission. More than 3 years of semaglutide therapy improved the patients’ metabolic control of diabetes and resulted in significant weight loss (16% of the initial body mass), with no reported adverse drug reactions and without compromising hematologic stability. Our case report suggests that hemato-oncological patients should not be categorically excluded from treatment with semaglutide, as long as close hematological and clinical monitoring is ensured. However, as this observation is based on a single case report, no definitive general recommendations regarding the safety of semaglutide in hemato-oncological patients can be made at this time.
REFERENCES (26)
1.
Kim D.S., Scherer P.E. Obesity, diabetes, and increased cancer progression. Diabetes Metab. J. 2021; 45(6): 799–812, doi: 10.4093/dmj.2021.0077.
2.
Scully T., Ettela A., LeRoith D., Gallagher E.J. Obesity, type 2 diabetes, and cancer risk. Front. Oncol. 2021; 10: 615375, doi: 10.3389/fonc.2020.615375.
3.
Pasupuleti S.K., Kapur R. The impact of obesity-induced inflammation on clonal hematopoiesis. Curr. Opin. Hematol. 2024; 31(4): 193–198, doi: 10.1097/MOH.0000000000000819.
4.
Zheng Z., Zong Y., Ma Y., Tian Y., Pang Y., Zhang C. et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024; 9(1): 234, doi: 10.1038/s41392-024-01931-z.
5.
Liu Z.Z., Duan X.X., Yuan M.C., Yu J., Hu X., Han X. et al. Glucagon-like peptide-1 receptor activation by liraglutide promotes breast cancer through NOX4/ROS/VEGF pathway. Life Sci. 2022; 294: 120370, doi: 10.1016/j.lfs.2022.120370.
6.
Mali G., Ahuja V., Dubey K. Glucagon-like peptide-1 analogues and thyroid cancer: An analysis of cases reported in the European pharmacovigilance database. J. Clin. Pharm. Ther. 2021; 46(1): 99–105, doi: 10.1111/jcpt.13259.
7.
Lisco G., De Tullio A., Disoteo O., Piazzolla G., Guastamacchia E., Sabbà C. et al. Glucagon-like peptide 1 receptor agonists and thyroid cancer: is it the time to be concerned? Endocr. Connect. 2023; 12(11): e230257, doi: 10.1530/EC-23-0257.
8.
Piccoli G.F., Mesquita L.A., Stein C., Aziz M., Zoldan M., Degobi N.A. et al. Do GLP-1 receptor agonists increase the risk of breast cancer? A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021; 106(3): 912–921, doi: 10.1210/clinem/dgaa891.
9.
Smits M.M., Van Raalte D.H. Safety of semaglutide [published correction appears in Front. Endocrinol. 2021; 12: 786732]. Front. Endocrinol. 2021; 12: 645563, doi: 10.3389/fendo.2021.645563.
10.
Piché M.E., Tchernof A., Després J.P. Obesity phenotypes, diabetes, and cardiovascular diseases [published correction appears in Circ. Res. 2020; 127(3): e107, doi: 10.1161/RES.0000000000000421]. Circ. Res. 2020; 126(11): 1477–1500, doi: 10.1161/CIRCRESAHA.120.316101.
11.
Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019; 92: 121–135, doi: 10.1016/j.metabol.2018.11.001.
12.
Lichtman M.A. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist 2010; 15(10): 1083–1101, doi: 10.1634/theoncologist.2010-0206.
13.
Aoyagi T., Terracina K.P., Raza A., Matsubara H., Takabe K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015; 7(4): 17–29, doi: 10.4251/wjgo.v7.i4.17.
14.
Bruggeman A.R., Kamal A.H., LeBlanc T.W., Ma J.D., Baracos V.E., Roeland E.J. Cancer cachexia: beyond weight loss. J. Oncol. Pract. 2016; 12(11): 1163–1171, doi: 10.1200/JOP.2016.016832.
15.
Ligibel J.A., Bohlke K., May A.M., Clinton S.K., Demark-Wahnefried W., Gilchrist S.C. et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J. Clin. Oncol. 2022; 40(22): 2491–2507, doi: 10.1200/JCO.22.00687.
16.
O’Connell F., O’Sullivan J. Help or hindrance: The obesity paradox in cancer treatment response. Cancer Lett. 2021; 522: 269–280, doi: 10.1016/j.canlet.2021.09.021.
17.
Assumpção J.A.F., Pasquarelli-do-Nascimento G., Duarte M.S.V., Bonamino M.H., Magalhães K.G. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 2022; 29(1): 12, doi: 10.1186/s12929-022-00796-0.
18.
Cleto A.S., Schirlo J.M., Beltrame M., Gomes V.H.O., Acras I.H., Neiverth G.S. et al. Semaglutide effects on safety and cardiovascular outcomes in patients with overweight or obesity: a systematic review and meta-analysis. Int. J. Obes. 2025; 49(1): 21–30, doi: 10.1038/s41366-024-01646-9.
19.
Colin I.M., Gérard K.M. Once-weekly 2.4 mg semaglutide for weight management in obesity: a game changer? touchREV. Endocrinol. 2022; 18(1): 35–42, doi: 10.17925/EE.2022.18.1.35.
20.
Bezin J., Gouverneur A., Pénichon M., Mathieu C., Garrel R., Hillaire-Buys D. et al. GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care 2023; 46(2): 384–390, doi: 10.2337/dc22-1148.
21.
Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 23-26 October 2023. European Medicines Agency, 27 October 2023 [online] https://www.ema.europa.eu/en/n... [accessed on 12 April 2024].
22.
Feier C.V.I., Vonica R.C., Faur A.M., Streinu D.R., Muntean C. Assessment of thyroid carcinogenic risk and safety profile of GLP1-RA semaglutide (ozempic) therapy for diabetes mellitus and obesity: A systematic literature review. Int. J. Mol. Sci. 2024; 25(8): 4346, doi: 10.3390/ijms25084346.
23.
Nagendra L., Bg H., Sharma M., Dutta D. Semaglutide and cancer: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023; 17(9): 102834, doi:10.1016/j.dsx.2023.102834.
24.
Ashruf O.S., Hundal J., Mushtaq A., Kaelber D.C., Anwer F., Singh A. Hematologic cancers among patients with type 2 diabetes prescribed GLP-1 receptor agonists. JAMA Netw. Open 2025; 8(3): e250802, doi: 10.1001/jamanetworkopen.2025.0802.
25.
Sørum M.E., Gang A.O., Tholstrup D.M., Gudbrandsdottir S., Kissow H., Kornblit B. et al. Semaglutide treatment for PRevention Of Toxicity in high-dosE Chemotherapy with autologous haematopoietic stem-cell Transplantation (PROTECT): study protocol for a randomised, double-blind, placebo-controlled, investigator-initiated study. BMJ Open 2024; 14(10): e089862, doi: 10.1136/bmjopen-2024-089862.
26.
Vainer N., Rotbain Curovic V., Niemann C.U., Slager S.L., Rotbain E.C. Understanding the interplay between chronic lymphocytic leukemia and type 2 diabetes. Expert Rev. Hematol. 2024; 17(9): 617–629, doi: 10.1080/17474086.2024.2383417.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.