Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
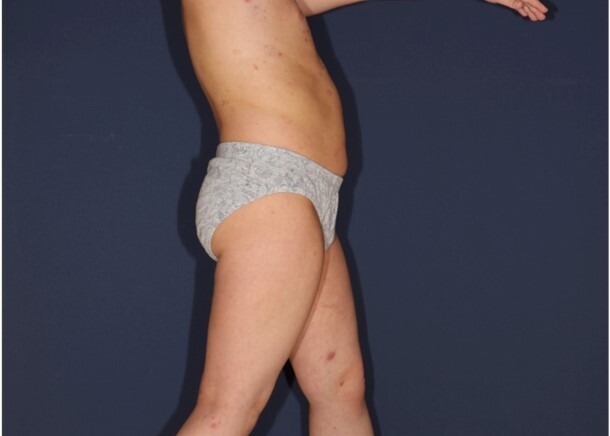
Pediatric lymphomatoid papulosis – a case report and management considerations
1
Department of Dermatology and Vascular Anomalies, Center of Pediatrics John Paul II, Sosnowiec
These authors had equal contribution to this work
Corresponding author
Michał Dec
Oddział Dermatologii i Leczenia Anomalii Naczyniowych dla Dzieci, Centrum Pediatrii im. Jana Pawła II w Sosnowcu Sp. z o.o., ul. G. Zapolskiej 3, 41-218 Sosnowiec
Oddział Dermatologii i Leczenia Anomalii Naczyniowych dla Dzieci, Centrum Pediatrii im. Jana Pawła II w Sosnowcu Sp. z o.o., ul. G. Zapolskiej 3, 41-218 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:24-30
KEYWORDS
lymphomatoid papulosisCD30-positive lymphoproliferative disorderphototherapyimmunohistochemistrymethotrexate
TOPICS
ABSTRACT
Lymphomatoid papulosis (LyP) is a rare cutaneous disorder, most commonly observed in adults, and its occurrence in the pediatric population is exceedingly rare. We present the case of an 6-year-old male patient who exhibited clinical and histopathological features consistent with LyP. The patient presented with multiple erythematous papules on the trunk and extremities, which were accompanied by mild pruritus. The lesions intermittently appeared, disappeared, and changed in morphology. No lymphadenopathy or systemic symptoms were noted. The histopathological examination revealed a dense infiltrate of atypical lymphocytes with cerebriform nuclei in the dermis. Immunohistochemical analysis confirmed CD30 expression in the infiltrating cells, supporting the diagnosis of LyP.
Topical corticosteroids were administered to alleviate pruritus and inflammation, although only minimal symptomatic relief was achieved. The beneficial effects of narrowband ultraviolet B (UVB) 311 phototherapy were observed for a duration of four months. Nevertheless, following the cessation of treatment, the reappearance of both the nodular lesions and smaller papular lesions was observed. Consequently, a therapeutic regimen consisting of the administration of methotrexate at a dosage of 10 mg once per week was initiated.
The treatment of LyP varies depending on the severity of the lesions and the patient’s symptoms, treatment decisions need to be carefully weighed due to the relatively benign nature of the disease.
The diagnosis of LyP in pediatric patients is challenging because of its rarity and potential confusion with malignant lymphomas. Histopathology and immunohistochemistry play a pivotal role in distinguishing LyP from more aggressive entities.
REFERENCES (48)
1.
Wang L., Chen F., Zhao S., Wang X., Fang J., Zhu X. Lymphomatoid papulosis subtype C: A case report and literature review. Dermatol. Ther. 2021; 34(1): e14452, doi: 10.1111/dth.14452.
2.
Bekkenk M.W., Geelen F.A., van Voorst Vader P.C., Heule F., Geerts M.L., van Vloten W.A. et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000; 95(12): 3653–3661.
3.
Wieser I., Oh C.W., Talpur R., Duvic M. Lymphomatoid papulosis: Treatment response and associated lymphomas in a study of 180 patients. J. Am. Acad. Dermatol. 2016; 74(1): 59–67, doi: 10.1016/j.jaad.2015.09.013.
4.
Miquel J., Fraitag S., Hamel-Teillac D., Molina T., Brousse N., de Prost Y. et al. Lymphomatoid papulosis in children: a series of 25 cases. Br. J. Dermatol. 2014; 171(5): 1138–1146, doi: 10.1111/bjd.13061.
5.
Burg G., Kempf W., Haeffner A., Döbbeling U., Nestle F.O., Böni R. et al. From inflammation to neoplasia: new concepts in the pathogenesis of cutaneous lymphomas. Recent Results Cancer Res. 2002; 160: 271–280, doi: 10.1007/978-3-642-59410-6_32.
6.
Nowicka D., Mertowska P., Mertowski S., Hymos A., Forma A., Michalski A. et al. Etiopathogenesis, diagnosis, and treatment strategies for lymphomatoid papulosis with particular emphasis on the role of the immune system. Cells 2022; 11(22): 3697, doi: 10.3390/cells11223697.
7.
Namba H., Hamada T., Iwatsuki K. Human T-cell leukemia virus type 1-positive lymphomatoid papulosis. Eur. J. Dermatol. 2016; 26(2): 194–195, doi: 10.1684/ejd.2015.2707.
8.
Kempf W., Kadin M.E., Dvorak A.M., Lord C.C., Burg G., Letvin N.L. et al. Endogenous retroviral elements, but not exogenous retroviruses, are detected in CD30-positive lymphoproliferative disorders of the skin. Carcinogenesis 2003; 24(2): 301–306, doi: 10.1093/carcin/24.2.301.
9.
Kempf W., Kadin M.E., Kutzner H., Lord C.L., Burg G., Letvin N.L. et al. Lymphomatoid papulosis and human herpesviruses – A PCR-based evaluation for the presence of human herpesvirus 6, 7 and 8 related herpesviruses. J. Cutan. Pathol. 2001; 28(1): 29–33, doi: 10.1034/j.1600-0560.2001.280103.x.
10.
Hooper M.J., Lee W.J., LeWitt T.M., Nguyen C., Griffin T., Chung C. et al. Epstein-Barr virus-associated lymphomatoid papules: A sign of immunosuppression resembling lymphomatoid papulosis. Am. J. Dermatopathol. 2023; 45(12): 789–800, doi: 10.1097/DAD.0000000000002479.
11.
Kim Y.C., Yang W.I., Lee M.G., Kim S.N., Cho K.H., Lee S.J. et al. Epstein-Barr virus in CD30 anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int. J. Dermatol. 2006; 45(11): 1312–1316, doi: 10.1111/j.1365-4632.2006.02951.x.
12.
Zhao P., Gish T.J., Mannschreck D., Marchi E., Cropley T.G. Zosteriform mycosis fungoides and lymphomatoid papulosis arising in an area of prior herpes zoster. JAAD Case Rep. 2023; 40: 84–88, doi: 10.1016/j.jdcr.2023.08.018.
13.
Schiemann W.P., Pfeifer W.M., Levi E., Kadin M.E., Lodish H.F. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood 1999; 94(8): 2854–2861.
14.
Haro R., Juarez A., Díaz J.L., Santonja C., Manzarbeitia F., Requena L. Regional lymphomatoid papulosis of the breast restricted to an area of prior radiotherapy. Cutis. 2016; 97(5): E15–19.
15.
Samaraweera A.P.R., Cohen S.N., Akay E.M., Evangelou N. Lymphomatoid papulosis: A cutaneous lymphoproliferative disorder in a patient on fingolimod for multiple sclerosis. Mult. Scler. 2016; 22(1): 122–124, doi: 10.1177/1352458515597568.
16.
Park J.H., Lee J., Lee J.H., Lee D.Y., Koh E.M. Lymphomatoid papulosis in a patient treated with adalimumab for juvenile rheumatoid arthritis. Dermatology 2012; 225(3): 259–263, doi: 10.1159/000345104.
17.
Matoula T., Nikolaou V., Marinos L., Katsavos S., Nasis G., Economidi A. et al. Lymphomatoid papulosis type D in a fingolimod-treated multiple sclerosis patient. Mult. Scler. 2016; 22(12): 1630–1631, doi: 10.1177/1352458516642032.
18.
Shirani A., Dalton S.R., Avery E.J., Arcot Jayagopal L., Meyer C., Stuve O. et al. Lymphomatoid papulosis in a patient treated with glatiramer acetate and the glatiramoid Glatopa for multiple sclerosis: A case report. J. Cent. Nerv. Syst. Dis. 2021; 13: 11795735211053784, doi: 10.1177/11795735211053784.
19.
Outlaw W., Fleischer A., Bloomfeld R. Lymphomatoid papulosis in a patient with Crohnʼs disease treated with infliximab. Inflamm. Bowel Dis. 2009; 15(7): 965–966, doi: 10.1002/ibd.20762.
20.
Leo A.M., Ermolovich T. Lymphomatoid papulosis while on efalizumab. J. Am. Acad. Dermatol. 2009; 61(3): 540–541, doi: 10.1016/j.jaad.2008.12.010.
21.
Stoll J.R., Willner J., Oh Y., Pulitzer M., Moskowitz A., Horwitz S. et al. Primary cutaneous T-cell lymphomas other than mycosis fungoides and Sézary syndrome. Part I: Clinical and histologic features and diagnosis. J. Am. Acad. Dermatol. 2021; 85(5): 1073–1090, doi: 10.1016/j.jaad.2021.04.080.
22.
Korpusik D., Ruzicka T. Klinische Verlaufsformen und Therapie der lymphomatoiden Papulose. Hautarzt 2007; 58(10): 870–881.
23.
Moy A., Sun J., Ma S., Seminario-Vidal L. Lymphomatoid papulosis and other lymphoma-like diseases. Dermatol. Clin. 2019; 37(4): 471–482, doi: 10.1016/j.det.2019.05.005.
24.
Pomsoong C., Suchonwanit P., Chanprapaph K., Rattanakaemakorn P., Rutnin S. Pityriasis lichenoides et varioliformis acuta and lymphomatoid papulosis type F: A case report of two entities in one patient. Clin. Cosmet. Investig. Dermatol. 2022; 15: 1759–1765, doi: 10.2147/CCID.S379577.
25.
Fujimura T., Lyu C., Tsuchiyama K., Aiba S. CD30-positive angioinvasive lymphomatoid papulosis (type E) developing from parapsoriasis en plaque. Case Rep. Oncol. 2018; 11(3): 850–854, doi: 10.1159/000495689.
26.
Heald P., Subtil A., Breneman D., Wilson L.D. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2007; 57(6): 1005–1011, doi: 10.1016/j.jaad.2007.05.046.
27.
Steinhoff M., Assaf C., Sterry W. Persistent agmination of lymphomatoid papulosis: not a new entity, but localized lymphomatoid papulosis. J. Am. Acad. Dermatol. 2008; 59(1): 164–165; author reply 165, doi: 10.1016/j.jaad.2007.12.039.
28.
Martinez-Cabriales S.A., Walsh S., Sade S., Shear N.H. Lymphomatoid papulosis: an update and review. J. Eur. Acad. Dermatol. Venereol. 2020; 34(1): 59–73, doi: 10.1111/jdv.15931.
29.
Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20): 2375–2390, doi: 10.1182/blood-2016-01-643569.
30.
Kempf W., Kazakov D.V., Schärer L., Rütten A., Mentzel T., Paredes B.E. et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am. J. Surg. Pathol. 2013; 37(1): 1–13, doi: 10.1097/PAS.0b013e3182648596.
31.
Saggini A., Gulia A., Argenyi Z., Fink-Puches R., Lissia A., Magaña M. et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Description of 9 cases. Am. J. Surg. Pathol. 2010; 34(8): 1168–1175, doi: 10.1097/PAS.0b013e3181e75356.
32.
Ross N.A., Truong H., Keller M.S., Mulholland J.K., Lee J.B., Sahu J. Follicular lymphomatoid papulosis: An eosinophilic-rich follicular subtype masquerading as folliculitis clinically and histologically. Am. J. Dermatopathol. 2016; 38(1): e1–10, doi: 10.1097/DAD.0000000000000395.
33.
Gheucă Solovăstru L., Vâţă D., Ciobanu D., Stătescu L., Rotaru M. The importance of histopathology findings in lymphomatoid papulosis. Rom. J. Morphol. Embryol. 2014; 55(4): 1527–1530.
34.
Werner B., Massone C., Kerl H., Cerroni L. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J. Cutan. Pathol. 2008; 35(12): 1100–1107, doi: 10.1111/j.1600-0560.2007.00979.x.
35.
Abdulla F.R., Zhang W., Wu X., Honda K., Qin H., Cho H. et al. Genomic analysis of cutaneous CD30-positive lymphoproliferative disorders. JID Innov. 2021; 2(1): 100068, doi: 10.1016/j.xjidi.2021.100068.
36.
Nikolaenko L., Zain J., Rosen S.T., Querfeld C. CD30-positive lymphoproliferative disorders. Cancer Treat. Res. 2019; 176: 249–268, doi: 10.1007/978-3-319-99716-2_12.
37.
Kempf W., Mitteldorf C., Karai L.J., Robson A. Lymphomatoid papulosis – making sense of the alphabet soup: a proposal to simplify terminology. J. Dtsch. Dermatol. Ges. 2017; 15(4): 390–394, doi: 10.1111/ddg.13207.
38.
Prieto-Torres L., Rodriguez-Pinilla S.M., Onaindia A., Ara M., Requena L., Piris M.Á. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica 2019; 104(2): 226–235, doi: 10.3324/haematol.2018.197152.
39.
Nijsten T., Curiel-Lewandrowski C., Kadin M.E. Lymphomatoid papulosis in children: a retrospective cohort study of 35 cases. Arch. Dermatol. 2004; 140(3): 306–312, doi: 10.1001/archderm.140.3.306.
40.
de Souza A., Camilleri M.J., Wada D.A., Appert D.L., Gibson L.E., el-Azhary R.A. Clinical, histopathologic, and immunophenotypic features of lymphomatoid papulosis with CD8 predominance in 14 pediatric patients. J. Am. Acad. Dermatol. 2009; 61(6): 993–1000, doi: 10.1016/j.jaad.2009.05.014.
41.
Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J. Cutan. Pathol. 2006; 33 Suppl 1: 58–70, doi: 10.1111/j.0303-6987.2006.00548.x.
42.
Thomsen K., Wantzin G.L. Lymphomatoid papulosis. A follow-up study of 30 patients. J. Am. Acad. Dermatol. 1987; 17(4): 632–636, doi: 10.1016/s0190-9622(87)70248-5.
43.
Everett M.A. Treatment of lymphomatoid papulosis with methotrexate. Br. J. Dermatol. 1984; 111(5): 631, doi: 10.1111/j.1365-2133.1984.tb06640.x.
44.
Vonderheid E.C., Sajjadian A., Kadin M.E. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J. Am. Acad. Dermatol. 1996; 34(3): 470–481, doi: 10.1016/s0190-9622(96)90442-9.
45.
Bruijn M.S., Horváth B., van Voorst Vader P.C., Willemze R., Vermeer M.H. Recommendations for treatment of lymphomatoid papulosis with methotrexate: a report from the Dutch Cutaneous Lymphoma Group. Br. J. Dermatol. 2015; 173(5): 1319–1322, doi: 10.1111/bjd.13920.
46.
Newland K.M., McCormack C.J., Twigger R., Buelens O., Hughes C.F.M., Lade S. et al. The efficacy of methotrexate for lymphomatoid papulosis. J. Am. Acad. Dermatol. 2015; 72(6): 1088–1090, doi: 10.1016/j.jaad.2015.03.001. Erratum in: J. Am. Acad. Dermatol. 2015; 73(3): 532.
47.
Champagne T., Walsh S. Mycophenolic acid for lymphomatoid papulosis. J. Cutan. Med. Surg. 2013; 17(5): 332–334, doi: 10.2310/7750.2013.12111.
48.
Duvic M., Tetzlaff M.T., Gangar P., Clos A.L., Sui D., Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J. Clin. Oncol. 2015; 33(32): 3759–3765, doi: 10.1200/JCO.2014.60.3787.
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.