Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
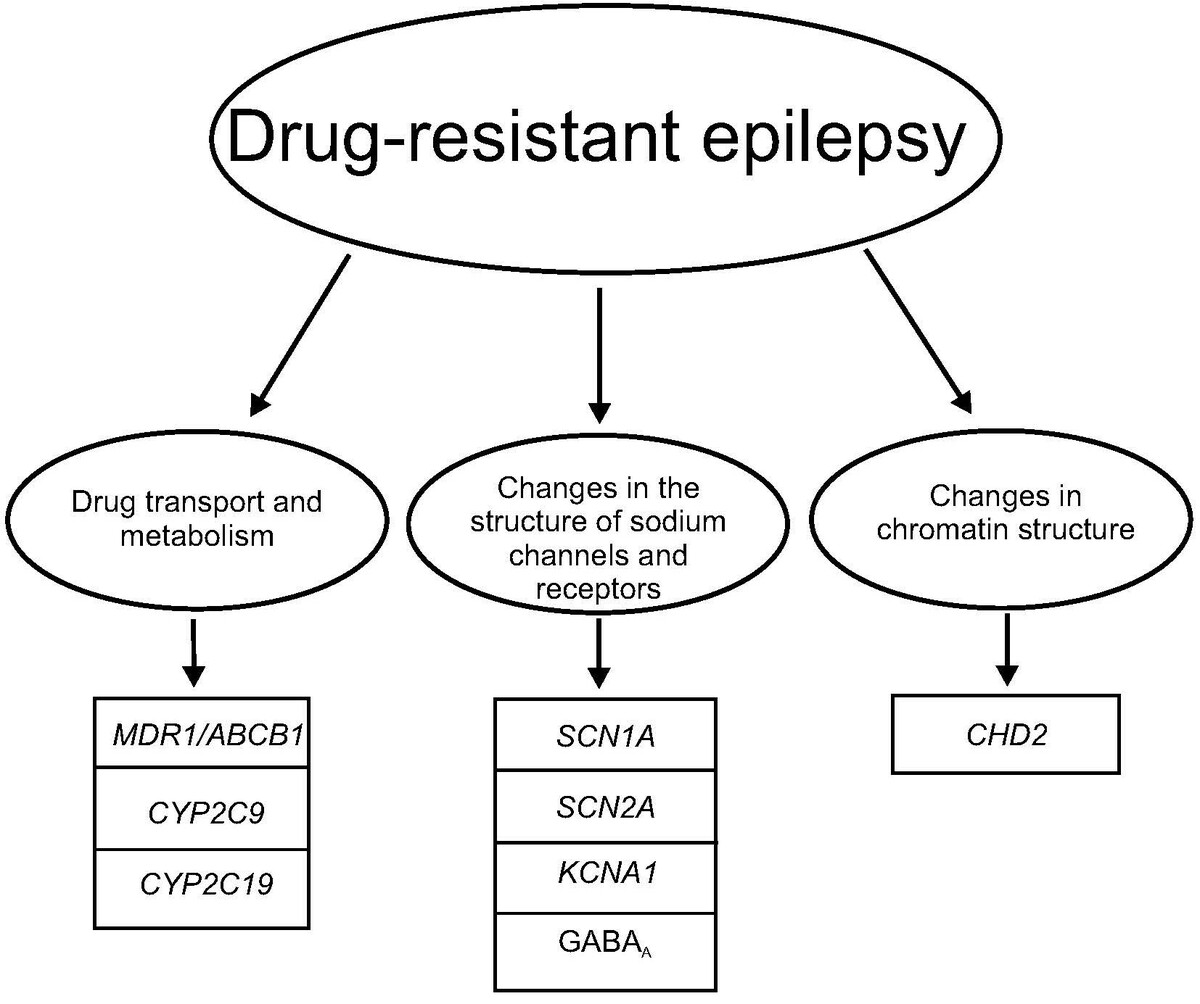
Drug-resistant epilepsy and its selected complications in children
1
Uzdrowisko Ustroń, Grupa American Heart of Poland, Ustroń, Poland
2
Department of Biochemistry and Medical Genetics, Faculty of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland
3
Department of Gastroenterology and Hepatology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Corresponding author
Joanna Iwanicka
Zakład Biochemii i Genetyki Medycznej, Wydział Nauk o Zdrowiu w Katowicach, Śląski Uniwersytet Medyczny w Katowicach, ul. Medyków 18, 40-752 Katowice
Zakład Biochemii i Genetyki Medycznej, Wydział Nauk o Zdrowiu w Katowicach, Śląski Uniwersytet Medyczny w Katowicach, ul. Medyków 18, 40-752 Katowice
Ann. Acad. Med. Siles. 2024;78:127-137
KEYWORDS
TOPICS
ABSTRACT
Drug-resistant epilepsy (DRE) in children is one of the most diagnosed disorders of the nervous system. Despite the availability of pharmacotherapy with various mechanisms of action, drug resistance leads to the inability to achieve the intended therapeutic effect. Considering the complex etiology of this disease entity, there is an urgent need to deepen our knowledge regarding the determinants of DRE, as well as the potential for improving the quality of life for patients and their families. This paper provides an overview and description of the latest advancements and current state of knowledge related to the clinical characteristics, epidemiology, possible complications, and especially genetic basis of DRE in the Polish population of children.
FUNDING
This work was conducted without external funding sources.
REFERENCES (104)
1.
GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18(4): 357–375, doi: 10.1016/S1474-4422(18)30454-X.
2.
Zack M.M., Kobau R. National and state estimates of the numbers of adults and children with active epilepsy – United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2017; 66(31): 821–825, doi: 10.15585/mmwr.mm6631a1.
3.
Fattorusso A., Matricardi S., Mencaroni E., Dell’Isola G.B., Di Cara G., Striano P. et al. The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front. Neurol. 2021; 12: 674483, doi: 10.3389/fneur.2021.674483.
4.
Eriksson K.J., Koivikko M.J. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia 1997; 38(12): 1275–1282, doi: 10.1111/j.1528-1157.1997.tb00064.x.
5.
Picot M.C., Baldy-Moulinier M., Daurès J.P., Dujols P., Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 2008; 49(7): 1230–1238, doi: 10.1111/j.1528-1167.2008.01579.x.
6.
Kun L.N., Ling L.W., Wah Y.W., Lian T.T. Epidemiologic study of epilepsy in young Singaporean men. Epilepsia 1999; 40(10): 1384–1387, doi: 10.1111/j.1528-1157.1999.tb02009.x.
7.
Farghaly W.M.A., El-Tallawy H.N., Rageh T.A., Mohamed E.M., Metwally N.A., Shehata G.A. et al. Epidemiology of uncontrolled epilepsy in the Al-Kharga District, New Valley, Egypt. Seizure 2013; 22(8): 611–616, doi: 10.1016/j.seizure.2013.04.010.
8.
Kontna E., Lewicka M., Małecka B., Barczykowska E. Clinical, therapeutic and caring aspects of epilepsy at the developmental age. JNNN 2016; 5(1): 31–35, doi: 10.15225/PNN.2016.5.1.6.
9.
Słowińska M., Jóźwiak S. History of advances in the diagnosis and treatment of epilepsy and new challenges of modern epileptology. [Article in Polish]. Child Neurol. 2017; 26(53): 11–17, doi: 10.20966/chn.2017.53.405.
10.
Löscher W., Potschka H., Sisodiya S.M., Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020; 72(3): 606–638, doi: 10.1124/pr.120.019539.
11.
Bazhanova E.D., Kozlov A.A., Litovchenko A.V. Mechanisms of drug resistance in the pathogenesis of epilepsy: role of neuroinflammation. A literature review. Brain Sci. 2021; 11(5): 663, doi: 10.3390/brainsci11050663.
12.
Kocamaz H., Işıkay S. Epilepsy and GI disorders [Internet]. In: Epilepsy – Advances in Diagnosis and Therapy. London, IntechOpen; 2019, doi: 10.5772/intechopen.85813.
13.
Krishnan V. Depression and anxiety in the epilepsies: from bench to bedside. Curr. Neurol. Neurosci. Rep. 2020; 20(9): 41, doi: 10.1007/s11910-020-01065-z.
14.
Oliva C.F., Gangi G., Marino S., Marino L., Messina G., Sciuto S. et al. Single and in combination antiepileptic drug therapy in children with epilepsy: how to use it. AIMS Med. Sci. 2021; 8(2): 138–146, doi: 10.3934/medsci.2021013.
15.
Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G. et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51(6): 1069–1077, doi: 10.1111/j.1528-1167.2009.02397.x.
16.
Misiurewicz-Gabi A., Słowik A., Bosak M. Padaczkę – także lekooporną – można leczyć. Kurier Medyczny 2022; https://www.termedia.pl/mz/Pad....
17.
Camfield P.R., Camfield C.S. Antiepileptic drug therapy: when is epilepsy truly intractable? Epilepsia 1996; 37 Suppl 1: 60–65, doi: 10.1111/j.1528-1157.1996.tb06023.x.
19.
Steinborn B., Mazurkiewicz-Bełdzińska M., Winczewska-Wiktor A. Padaczka i drgawki w różnych okresach życia dziecka. In: Steinborn B. [ed.]. Neurologia wieku rozwojowego. Wyd. Lekarskie PZWL. Warszawa 2017, p. 334–338.
20.
Kalilani L., Sun X., Pelgrims B., Noack-Rink M., Villanueva V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018; 59(12): 2179–2193, doi: 10.1111/epi.14596.
21.
Rola R. Zastosowanie głębokiej stymulacji mózgu jako alternatywa w leczeniu padaczki lekoopornej. Neurol. Prakt. 2012; 2(65): 7–10.
22.
Tang F., Hartz A.M.S., Bauer B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front. Neurol. 2017; 8: 301, doi: 10.3389/fneur.2017.00301.
23.
Lazarowski A., Czornyj L., Lubienieki F., Girardi E., Vazquez S., D’Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 2007; 48 Suppl 5: 140–149, doi: 10.1111/j.1528-1167.2007.01302.x.
24.
Perucca E., Bialer M., White H.S. New GABA-targeting therapies for the treatment of seizures and epilepsy: I. Role of GABA as a modulator of seizure activity and recently approved medications acting on the GABA system. CNS Drugs 2023; 37(9): 755–779, doi: 10.1007/s40263-023-01027-2.
25.
Fang M., Xi Z.Q., Wu Y., Wang X.F. A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med. Hypotheses 2011; 76(6): 871–876, doi: 10.1016/j.mehy.2011.02.039.
26.
Rogawski M.A., Johnson M.R. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 2008; 8(5): 127–130, doi: 10.1111/j.1535-7511.2008.00272.x.
27.
Depondt C. The potential of pharmacogenetics in the treatment of epilepsy. Eur. J. Paediatr. Neurol. 2006; 10(2): 57–65, doi: 10.1016/j.ejpn.2005.11.009.
28.
Remy S., Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2006; 129(Pt 1): 18–35, doi: 10.1093/brain/awh682.
29.
Löscher W., Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005; 6(8): 591–602, doi: 10.1038/nrn1728.
30.
Feldmann M., Asselin M.C., Liu J., Wang S., McMahon A., Anton-Rodriguez J. et al. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013; 12(8): 777–785, doi: 10.1016/S1474-4422(13)70109-1.
31.
Kobow K., El-Osta A., Blümcke I. The methylation hypothesis of pharmacoresistance in epilepsy. Epilepsia 2013; 54(Suppl 2): 41–47, doi: 10.1111/epi.12183.
32.
Kobow K., Blümcke I. Epigenetics in epilepsy. Neurosci. Lett. 2018; 667: 40–46, doi: 10.1016/j.neulet.2017.01.012.
33.
Wang J., Lin Z.J., Liu L., Xu H.Q., Shi Y.W., Yi Y.H. et al. Epilepsy-associated genes. Seizure 2017; 44: 11–20, doi: 10.1016/j.seizure.2016.11.030.
34.
Mei D., Cetica V., Marini C., Guerrini R. Dravet syndrome as part of the clinical and genetic spectrum of sodium channel epilepsies and encephalopathies. Epilepsia 2019; 60 Suppl 3: 2–7, doi: 10.1111/epi.16054.
35.
Brunklaus A., Ellis R., Stewart H., Aylett S., Reavey E., Jefferson R. et al. Homozygous mutations in the SCN1A gene associated with genetic epilepsy with febrile seizures plus and Dravet syndrome in 2 families. Eur. J. Paediatr. Neurol. 2015; 19(4): 484–488, doi: 10.1016/j.ejpn.2015.02.001.
36.
Szczepanik E., Terczyńska I., Hoffman-Zacharska D. SCN1A-related spectrum of generalized epilepsy syndromes and epilepsy with febrile seizures plus. [Article in Polish]. Neurol. Dziec. 2009; 18(36): 41–46.
37.
Margari L., Legrottaglie A.R., Vincenti A., Coppola G., Operto F.F., Buttiglione M. et al. Association between SCN1A gene polymorphisms and drug resistant epilepsy in pediatric patients. Seizure 2018; 55: 30–35, doi: 10.1016/j.seizure.2018.01.002.
38.
Liu M., Mao J., Xu H., Wang J., Zhao P., Xu Q. et al. Effects of SCN1A and SCN2A polymorphisms on responsiveness to valproic acid monotherapy in epileptic children. Epilepsy Res. 2020; 168: 106485, doi: 10.1016/j.eplepsyres.2020.106485.
39.
Nazish H.R., Ali N., Ullah S. The possible effect of SCN1A and SCN2A genetic variants on carbamazepine response among Khyber Pakhtunkhwa epileptic patients, Pakistan. Ther. Clin. Risk Manag. 2018; 14: 2305–2313, doi: 10.2147/TCRM.S180827.
40.
Yang X., Yan Y., Fang S., Zeng S., Ma H., Qian L. et al. Comparison of oxcarbazepine efficacy and MHD concentrations relative to age and BMI: Associations among ABCB1, ABCC2, UGT2B7, and SCN2A polymorphisms. Medicine (Baltimore) 2019; 98(12): e14908, doi: 10.1097/MD.0000000000014908.
41.
Li X., Zhang J., Wu X., Yan H., Zhang Y., He R.H. et al. Polymorphisms of ABAT, SCN2A and ALDH5A1 may affect valproic acid responses in the treatment of epilepsy in Chinese. Pharmacogenomics 2016; 17(18): 2007–2014, doi: 10.2217/pgs-2016-0093.
42.
Trivisano M., Striano P., Sartorelli J., Giordano L., Traverso M., Accorsi P. et al. CHD2 mutations are a rare cause of generalized epilepsy with myoclonic–atonic seizures. Epilepsy Behav. 2015; 51: 53–56, doi: 10.1016/j.yebeh.2015.06.029.
43.
Nieto-Estevez V., Hsieh J. CHD2: one gene, many roles. Neuron 2018; 100(5): 1014–1016, doi: 10.1016/j.neuron.2018.11.036.
44.
Chen J., Zhang J., Liu A., Zhang L., Li H., Zeng Q. et al. CHD2‐related epilepsy: novel mutations and new phenotypes. Dev. Med. Child. Neurol. 2020; 62(5): 647–653, doi: 10.1111/dmcn.14367.
45.
Thomas R.H., Zhang L.M., Carvill G.L., Archer J.S., Heavin S.B., Mandelstam S.A. et al. CHD2 myoclonic encephalopathy is frequently associated with self-induced seizures. Neurology 2015; 84(9): 951–958, doi: 10.1212/WNL.0000000000001305.
46.
Miller D.S., Bauer B., Hartz A.M.S. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 2008; 60(2): 196–209, doi: 10.1124/pr.107.07109.
47.
Lv R.J., Shao X.Q., Cui T., Wang Q. Significance of MDR1 gene C3435T polymorphism in predicting childhood refractory epilepsy. Epilepsy Res. 2017; 132: 21–28, doi: 10.1016/j.eplepsyres.2017.02.010.
48.
Stasiołek M., Romanowicz H., Połatyńska K., Chamielec M., Skalski D., Makowska M. et al. Association between C3435T polymorphism of MDR1 gene and the incidence of drug-resistant epilepsy in the population of Polish children. Behav. Brain Funct. 2016; 12(1): 21, doi: 10.1186/s12993-016-0106-z.
49.
Zhao T., Li H.J., Yu J., Zhang H.L., Feng J., Wang T.T. et al. ABCB1 gene polymorphisms are closely associated with drug-resistant epilepsy: evidence based on 377 subjects in Chinese pediatric patients. Clin. Neuropharmacol. 2023; doi: 10.1097/WNF.0000000000000555.
50.
Chouchi M., Klaa H., Ben-Youssef Turki I., Hila L. ABCB1 Polymorphisms and drug-resistant epilepsy in a Tunisian population. Dis. Markers 2019; 2019: 1343650, doi: 10.1155/2019/1343650.
51.
Makowska M., Smolarz B., Bryś M., Forma E., Romanowicz H. An association between the rs1799853 and rs1057910 polymorphisms of CYP2C9, the rs4244285 polymorphism of CYP2C19 and the prevalence rates of drug-resistant epilepsy in children. Int. J. Neurosci. 2021; 131(12): 1147–1154, doi: 10.1080/00207454.2020.1781110.
52.
Ochoa J.G., Kilgo W.A. The role of benzodiazepines in the treatment of epilepsy. Curr. Treat. Options Neurol. 2016; 18(4): 18, doi: 10.1007/s11940-016-0401-x.
53.
Chakraborty A., Dey S., Kumar K., Dixit A.B., Tripathi M., Sharma M.C. et al. Novel variants in GABAA receptor subunits: A possible association with benzodiazepine resistance in patients with drug-resistant epilepsy. Epilepsy Res. 2023; 189: 107056, doi: 10.1016/j.eplepsyres.2022.107056.
54.
Savic I., Persson A., Roland P., Pauli S., Sedvall G., Widén L. In-vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci. Lancet 1988; 2(8616): 863–866, doi: 10.1016/s0140-6736(88)92468-3.
55.
Baulac S., Huberfeld G., Gourfinkel-An I., Mitropoulou G., Beranger A., Prud’homme J.F. et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat. Genet. 2001; 28(1): 46–48, doi: 10.1038/ng0501-46.
56.
Wallace R.H., Marini C., Petrou S., Harkin L.A., Bowser D.N., Panchal R.G. et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001; 28(1): 49–52, doi: 10.1038/ng0501-49.
57.
Maljevic S., Keren B., Aung Y.H., Forster I.C., Mignot C., Buratti J. et al. Novel GABRA2 variants in epileptic encephalopathy and intellectual disability with seizures. Brain 2019; 142(5): e15, doi: 10.1093/brain/awz079.
58.
Cossette P., Liu L., Brisebois K., Dong H., Lortie A., Vanasse M. et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 2002; 31(2): 184–189, doi: 10.1038/ng885.
59.
Kapoor A., Vijai J., Ravishankar H.M., Satishchandra P., Radhakrishnan K., Anand A. Absence of GABRA1 Ala322Asp mutation in juvenile myoclonic epilepsy families from India. J. Genet. 2003; 82(1–2): 17–21, doi: 10.1007/BF02715876.
60.
Butler K.M., Moody O.A., Schuler E., Coryell J., Alexander J.J., Jenkins A. et al. De novo variants in GABRA2 and GABRA5 alter receptor function and contribute to early-onset epilepsy. Brain 2018; 141(8): 2392–2405, doi: 10.1093/brain/awy171.
61.
Orenstein N., Goldberg-Stern H., Straussberg R., Bazak L., Weisz Hubshman M., Kropach N. et al. A de novo GABRA2 missense mutation in severe early-onset epileptic encephalopathy with a choreiform movement disorder. Eur. J. Paediatr. Neurol. 2018; 22(3): 516–524, doi: 10.1016/j.ejpn.2017.12.017.
62.
Flint H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012; 70 Suppl 1: S10–13, doi: 10.1111/j.1753-4887.2012.00499.x.
63.
Foster J.A., McVey Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013; 36(5): 305–312, doi: 10.1016/j.tins.2013.01.005.
64.
Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R. et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014; 26(8): 1155–1162, doi: 10.1111/nmo.12378.
65.
Holingue C., Newill C., Lee L.C., Pasricha P.J., Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res. 2018; 11(1): 24–36, doi: 10.1002/aur.1854.
66.
Song Y., Liu C., Finegold S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004; 70(11): 6459–6465, doi: 10.1128/AEM.70.11.6459-6465.2004.
67.
Grice E.A, Segre J.A. The human microbiome: our second genome. Annu. Rev. Genomics Hum. Genet. 2012; 13: 151–170, doi: 10.1146/annurev-genom-090711-163814.
68.
Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010; 107(26): 11971–11975, doi: 10.1073/pnas.1002601107.
69.
Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017; 8(32): 53829–53838, doi: 10.18632/oncotarget.17754.
70.
Peng A., Qiu X., Lai W., Li W., Zhang L., Zhu X. et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 2018; 147: 102–107, doi: 10.1016/j.eplepsyres.2018.09.013.
71.
Shaikh M.F., Lee C.Y., Chen W.N., Shaikh F.A. The gut-brain-axis on the manifestation of depressive symptoms in epilepsy: an evidence-driven hypothesis. Front. Pharmacol. 2020; 11: 465, doi: 10.3389/fphar.2020.00465.
72.
Deng X., Xie Y., Chen Y. Effect of neuroinflammation on ABC transporters: possible contribution to refractory epilepsy. CNS Neurol. Disord. Drug Targets 2018; 17(10): 728–735, doi: 10.2174/1871527317666180828121820.
73.
Bhatia S., Schmitt S.E. Treating immune-related epilepsy. Curr. Neurol. Neurosci. Rep. 2018; 18(3): 10, doi: 1007/s11910-018-0821-y.
74.
Geis C., Planagumà J., Carreño M., Graus F., Dalmau J. Autoimmune seizures and epilepsy. J. Clin. Invest. 2019; 129(3): 926–940, doi: 10.1172/JCI125178.
75.
Dalmau J., Geis C., Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 2017; 97(2): 839–887, doi: 10.1152/physrev.00010.2016.
76.
Służewski W., Służewska-Niedźwiedź M. Uwarunkowania diagno-styczno-terapeutyczne w padaczce wieku rozwojowego. Pol. Przegl. Neurol. 2010; 6(3): 121–130.
77.
Rejdak K., Rola R., Mazurkiewicz-Bełdzińska M., Halczuk I., Błaszczyk B., Rysz A. et al. Diagnostyka i leczenie padaczki u osób dorosłych – rekomendacje Polskiego Towarzystwa Neurologicznego. Pol. Przegl. Neurol. 2016; 12(1): 15–27.
78.
Hoffman-Zacharska D. Epileptic encephalopathies – next generation diagnostics. [Article in Polish]. Child Neurol. 2017; 26(52): 75–83, doi: 10.20966/chn.2017.52.396.
79.
Jóźwiak S., Kotulska K. Current recommendations in the treatment of epilepsy and epileptic syndromes in children and adolescents. [Article in Polish]. Neurol. Dziec. 2010; 19(38): 11–18.
80.
Yıldız E.P., Hızlı Z., Bektaş G., Ulak-Özkan M., Tatlı B., Aydınlı N. et al. Efficacy of rufinamide in childhood refractory epilepsy. Turk. J. Pediatr. 2018; 60(3): 238–243, doi: 10.24953/turkjped.2018.03.002.
81.
Wykes R.C., Lignani G. Gene therapy and editing: Novel potential treatments for neuronal channelopathies. Neuropharmacology 2018; 132: 108–117, doi: 10.1016/j.neuropharm.2017.05.029.
82.
El Bahh B., Balosso S., Hamilton T., Herzog H., Beck-Sickinger A.G., Sperk G. et al. The anti‐epileptic actions of neuropeptide Y in the hippocampus are mediated by Y2 and not Y5 receptors. Eur. J. Neurosci. 2005; 22(6): 1417–1430, doi: 10.1111/j.1460-9568.2005.04338.x.
83.
Ledri L.N., Melin E., Christiansen S.H., Gøtzsche C.R., Cifra A., Woldbye D.P. et al. Translational approach for gene therapy in epilepsy: Model system and unilateral overexpression of neuropeptide Y and Y2 receptors. Neurobiol. Dis. 2016; 86: 52–61, doi: 10.1016/j.nbd.2015.11.014.
84.
Ledri M., Sørensen A.T., Madsen M.G., Christiansen S.H., Ledri L.N., Cifra A. et al. Differential effect of neuropeptides on excitatory synaptic transmission in human epileptic hippocampus. J. Neurosci. 2015; 35(26): 9622–9631, doi: 10.1523/JNEUROSCI.3973-14.2015.
85.
Snowball A., Chabrol E., Wykes R.C., Shekh-Ahmad T., Cornford J.H., Lieb A. et al. Epilepsy gene therapy using an engineered potassium channel. J. Neurosci. 2019; 39(16): 3159–3169, doi: 10.1523/JNEUROSCI.1143-18.2019.
86.
Paprocka J., Bacler-Żbikowska B. Cannabinoids in child neurology. [Article in Polish]. Child Neurol. 2015; 24(49): 79–87, doi: 10.20966/chn.2016.50.368.
87.
Chyra M., Mandera M., Dudzińska M., Mikanik-Klemens M. The Role of FDG PET/CT in qualification for neurosurgical treatment children with refractory epilepsy. [Article in Polish]. Child Neurol. 2016; 25(50): 19–26, doi: 10.20966/chn.2016.50.362.
88.
Karakas C., Houck K., Handoko M., Trandafir C., Coorg R., Haneef Z. et al. Responsive neurostimulation for the treatment of children with drug-resistant epilepsy in tuberous sclerosis complex. Pediatr. Neurol. 2023; 145: 97–101, doi: 10.1016/j.pediatrneurol.2023.05.008.
89.
Jastrzębski K. Ketogenic diet for epilepsy. [Article in Polish]. Aktualn. Neurol. 2017; 17(4): 214–219, doi: 10.15557/AN.2017.0024.
90.
Wells J., Swaminathan A., Paseka J., Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy: A review. Nutrients 2020; 12(6): 1809, doi: 10.3390/nu12061809.
91.
Park S., Lee E.J., Eom S., Kang H.C., Lee J.S., Kim H.D. Ketogenic diet for the management of epilepsy associated with tuberous sclerosis complex in children. J. Epilepsy Res. 2017; 7(1): 45–49, doi: 10.14581/jer.17008.
92.
Lemmon M.E., Terao N.N., Ng Y.T., Reisig W., Rubenstein J.E., Kossoff E.H. Efficacy of the ketogenic diet in Lennox–Gastaut syndrome: a retrospective review of one institution’s experience and summary of the literature. Dev. Med. Child Neurol. 2012; 54(5): 464–468, doi: 10.1111/j.1469-8749.2012.04233.x.
93.
Dressler A., Trimmel-Schwahofer P., Reithofer E., Mühlebner A., Gröppel G., Reiter-Fink E. et al. Efficacy and tolerability of the ketogenic diet in Dravet syndrome – comparison with various standard antiepilepticdrug regimen. Epilepsy Res. 2015; 109: 81–89, doi: 10.1016/j.eplepsyres.2014.10.014.
94.
Devi N., Madaan P., Kandoth N., Bansal D., Sahu J.K. Efficacy and safety of dietary therapies for childhood drug-resistant epilepsy: A systematic review and network meta-analysis. JAMA Pediatr. 2023; 177(3): 258–266, doi: 10.1001/jamapediatrics.2022.5648.
95.
Gómez-Eguílaz M., Ramón-Trapero J.L., Pérez-Martínez L., Blanco J.R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef. Microbes 2018; 9(6): 875–881, doi: 10.3920/BM2018.0018.
96.
Bagheri S., Heydari A., Alinaghipour A., Salami M. Effect of probiotic supplementation on seizure activity and cognitive performance in PTZ-induced chemical kindling. Epilepsy Behav. 2019; 95: 43–50, doi: 10.1016/j.yebeh.2019.03.038.
97.
Sillanpää M., Shinnar S. Long-term mortality in childhood-onset epilepsy. N. Engl. J. Med. 2010; 363(26): 2522–2529, doi: 10.1056/NEJMoa0911610.
98.
Camfield C., Camfield P. Injuries from seizures are a serious, persistent problem in childhood onset epilepsy: a population-based study. Seizure 2015; 27: 80–83, doi: 10.1016/j.seizure.2015.02.031.
99.
Suh J.I. Drug-induced liver injury. Yeungnam Univ. J. Med. 2020; 37(1): 2–12, doi: 10.12701/yujm.2019.00297.
100.
Chalasani N., Fontana R.J., Bonkovsky H.L., Watkins P.B., Davern T., Serrano J. et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135(6): 1924–1934, doi: 10.1053/j.gastro.2008.09.011.
101.
Bjornsson E., Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J. Hepatol. 2009; 50(3): 511–517, doi: 10.1016/j.jhep.2008.10.021.
102.
Reuben A., Koch D.G., Lee W.M.; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52(6): 2065–2076, doi: 10.1002/hep.23937.
103.
Elliott I.M., Lach L., Smith M.L. I just want to be normal: A qualitative study exploring how children and adolescents view the impact of intractable epilepsy on their quality of life. Epilepsy Behav. 2005; 7(4): 664–678, doi: 10.1016/j.yebeh.2005.07.004.
104.
Eklund P.G., Sivberg B. Adolescents’ lived experience of epilepsy. J. Neurosci. Nurs. 2003; 35(1): 40–49, doi: 10.1097/01376517-200302000-00008.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.