Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Selected adipokines as potential prognostic and diagnostic agents in treatment of metabolic disorders associated with obesity
1
Students’ Scientific Club, Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Students’ Scientific Club, Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
4
Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Corresponding author
Klaudia Stocerz
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:138-145
KEYWORDS
TOPICS
ABSTRACT
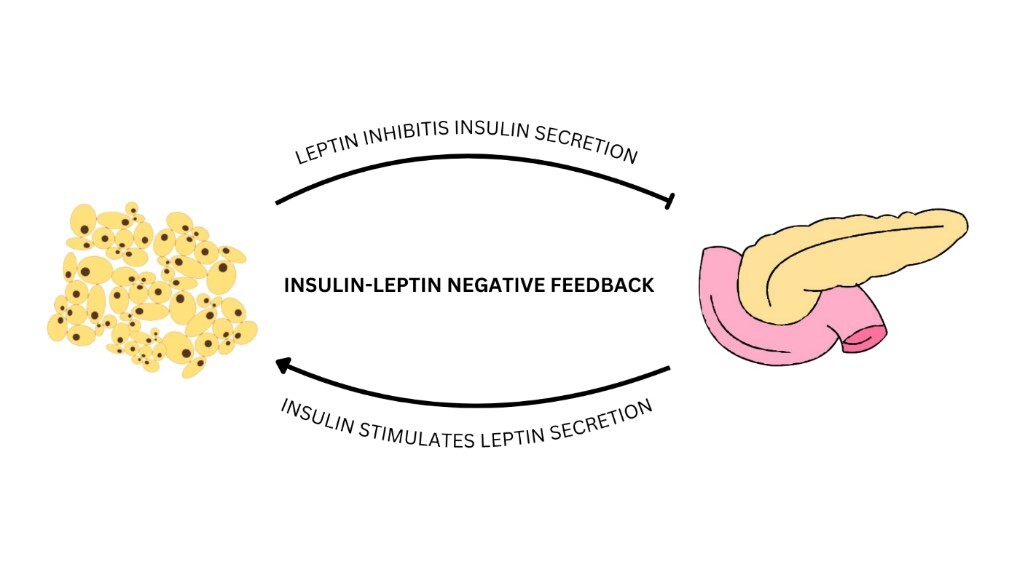
Obesity is a chronic disease that constitutes a global problem and a serious challenge to public health. In the course of this disease, excessive accumulation of fat tissue occurs in the body, which not only leads to an increased risk of health complications, but also negatively affects the quality of life. Fat cells – adipocytes – are responsible for the biosynthesis and release of adipokines, among others, leptin, visfatin, chemerin and omentin-1. They are active biological substances, including mediators of inflammation, which may lead to the development of metabolic disorders in the body. The above-mentioned properties of the described adipokines make it possible to potentially use them as diagnostic and therapeutic factors in the course of obesity and its accompanying disorders such as insulin resistance, type 2 diabetes mellitus, and cardiometabolic complications. Despite significant progress in understanding the role played by the discussed adipokines, further research is necessary to precisely describe the mechanisms of action and to determine the precise relationship between their plasma concentrations and state of diseases.
REFERENCES (52)
1.
Zatterale F., Longo M., Naderi J., Raciti G.A., Desiderio A., Miele C., Beguinot F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020; 10: 1607, doi: 10.3389/fphys.2019.01607.
2.
Longo M., Spinelli R., D’Esposito V., Zatterale F., Fiory F., Nigro C. et al. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim. Biophys. Acta 2016; 1863(6 Pt A): 1146–1156, doi: 10.1016/j.bbamcr.2016.02.019.
3.
Chen L., Chen R., Wang H., Liang F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015; 2015: 508409, doi: 10.1155/2015/508409.
4.
Ahmed B., Sultana R., Greene M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021; 137: 111315, doi: 10.1016/j.biopha.2021.111315.
5.
Ormazábal V., Nair S., Elfeky O., Aguayo C., Salomón C., Zúñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018; 17(1): 122, doi: 10.1186/s12933-018-0762-4.
6.
Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019; 234(6): 8152–8161, doi: 10.1002/jcp.27603.
7.
Li M., Chi X., Wang Y., Setrerrahmane S., Xie W., Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022; 7(1): 216, doi: 10.1038/s41392-022-01073-0.
8.
Sangwung P., Petersen K.F., Shulman G.I., Knowles J.W. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology 2020; 161(4): bqaa017, doi: 10.1210/endocr/bqaa017.
9.
Araszkiewicz A., Bandurska-Stankiewicz E., Borys S., Budzyński A., Cyganek K., Cypryk K. et al. 2023 Guidelines on the management of patients with diabetes – A position of Diabetes Poland. Curr. Top. Diabetes 2023; 3(1): 1–133, doi: 10.5114/ctd/160061.
10.
Harreiter J., Roden M. Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023) [Article in German]. Wien. Klin. Wochenschr. 2023; 135(Suppl 1): 7–17, doi: 10.1007/s00508-022-02122-y.
11.
Alam S., Hasan MdK., Neaz S., Hussain N., Hossain MdF., Rahman T. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021; 2(2): 36–50, doi: 10.3390/diabetology2020004.
12.
Liang W., Ye D.D. The potential of adipokines as biomarkers and therapeutic agents for vascular complications in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2019; 48: 32–39, doi: 10.1016/j.cytogfr.2019.06.002.
13.
Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch. Med. Sci. 2013; 9(2): 191–200, doi: 10.5114/aoms.2013.33181.
14.
Taylor E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021; 135(6): 731–752, doi: 10.1042/cs20200895.
15.
Obradović M., Sudar-Milovanović E., Šoškić S., Essack M., Arya S., Stewart A.J. et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. 2021; 12: 585887, doi: 10.3389/fendo.2021.585887.
16.
Coppari R., Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 2012; 11(9): 692–708, doi: 10.1038/nrd3757.
17.
Meek T.H., Morton G.J. The role of leptin in diabetes: metabolic effects. Diabetologia 2016; 59(5): 928–932, doi: 10.1007/s00125-016-3898-3.
18.
Moonishaa T.M., Nanda S.K., Shamraj M., Sivaa R., Sivakumar P., Ravichandran K. Evaluation of leptin as a marker of insulin resistance in type 2 diabetes mellitus. Int. J. Appl. Basic Med. Res. 2017; 7(3): 176–180, doi: 10.4103/ijabmr.ijabmr_278_16.
19.
Katsiki N., Mikhailidis D.P., Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018; 39(7): 1176–1188, doi: 10.1038/aps.2018.40.
20.
Kurajoh M., Koyama H., Kadoya M., Naka M., Miyoshi A., Kanzaki A. et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc. Diabetol. 2015; 14: 117, doi: 10.1186/s12933-015-0280-6.
21.
Morioka T., Emoto M., Yamazaki Y., Kawano N., Imamura S., Numaguchi R. et al. Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc. Diabetol. 2014; 13: 10, doi: 10.1186/1475-2840-13-10.
22.
Paz-Filho G., Mastronardi C., Wong M.L., Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 2012; 16(Suppl 3): S549–S555, doi: 10.4103/2230-8210.105571.
23.
Jung C.H., Kim B.Y., Mok J.O., Kang S.K., Kim C.H. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014; 5(3): 333–339, doi: 10.1111/jdi.12144.
24.
Vavruch C., Länne T., Fredrikson M., Lindström T., Östgren C.J., Nyström F.H. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc. Diabetol. 2015; 14: 62, doi: 10.1186/s12933-015-0208-1.
25.
El Husseny M.W., Mamdouh M., Shaban S., Ibrahim Abushouk A., Zaki M.M., Ahmed O.M. et al. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J. Diabetes Res. 2017; 2017: 8095926, doi: 10.1155/2017/8095926.
26.
Frühbeck G., Catalán V., Rodrı́guez A., Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018; 7(1): 57–62, doi: 10.1080/21623945.2017.1402151.
27.
Farr O.M., Gavrieli A., Mantzoros C.S. Leptin applications in 2015: what have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015; 22(5): 353–359, doi: 10.1097/med.0000000000000184.
28.
Zhao S., Zhu Y., Schultz R.D., Li N., He Z., Zhang Z. et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 2019; 30(4): 706–719.e6, doi: 10.1016/j.cmet.2019.08.005.
29.
Dakroub A., Nasser S.A., Younis N., Bhagani H., Al-Dhaheri Y., Pintus G. et al. Visfatin: a possible role in cardiovasculo-metabolic disorders. Cells 2020; 9(11): 2444, doi: 10.3390/cells9112444.
30.
Abdalla M.M.I. Role of visfatin in obesity-induced insulin resistance. World J. Clin. Cases 2022; 10(30): 10840–10851, doi: 10.12998/wjcc.v10.i30.10840.
31.
Hognogi L.D., Simiti L.V. The cardiovascular impact of visfatin – an inflammation predictor biomarker in metabolic syndrome. Clujul Med. 2016; 89(3): 322–326, doi: 10.15386/cjmed-591.
32.
Zheng L.Y., Xu X., Wan R.H., Sheng X., Lu J., Huang Q. Association between serum visfatin levels and atherosclerotic plaque in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2019; 11: 60, doi: 10.1186/s13098-019-0455-5.
33.
Uslu S., Kebapçı N., Kara M., Bal C. Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2012; 4(1): 113–120, doi: 10.3892/etm.2012.557.
34.
Ali S., Alam R., Ahsan H., Khan S. Role of adipokines (omentin and visfatin) in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2023; 33(3): 483–493, doi: 10.1016/j.numecd.2022.11.023.
35.
Léniz A., González M., Besné I., Carr-Ugarte H., Gómez-García I., Portillo M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022; 541: 111504, doi: 10.1016/j.mce.2021.111504.
36.
Fatima S.S., Butt Z., Bader N., Pathan A.Z., Hussain S., Iqbal N.T. Role of multifunctional Chemerin in obesity and preclinical diabetes. Obes. Res. Clin. Pract. 2015; 9(5): 507–512, doi: 10.1016/j.orcp.2015.01.004.
37.
Fatima S.S., Alam F., Chaudhry B., Khan T.A. Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2017; 30(9): 1023–1028, doi: 10.1080/14767058.2016.1199671.
38.
Zhou Z., Chen H., Ju H., Sun M. Circulating chemerin levels and gestational diabetes mellitus: a systematic review and meta-analysis. Lipids Health Dis. 2018; 17(1): 169, doi: 10.1186/s12944-018-0826-1.
39.
Yu S., Zhang Y., Li M.Z., Xu H., Wang Q., Song J. et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin. Med. J. (Engl) 2012; 125(19): 3440–3444, doi: 10.3760/cma.j.issn.0366-6999.2012.19.015.
40.
Mir M.M., Mir R., Alghamdi M.A.A., Wani J.I., Sabah Z.U., Jeelani M. et al. Differential association of selected adipocytokines, adiponectin, leptin, resistin, visfatin and chemerin, with the pathogenesis and progression of type 2 diabetes mellitus (T2DM) in the Asir region of Saudi Arabia: A case control study. J. Pers. Med. 2022; 12(5): 735, doi: 10.3390/jpm12050735.
41.
Waluga-Kozlowska E., Kuznik-Trocha K., Komosinska-Vassev K., Olczyk P., Jura-Poltorak A., Winsz-Szczotka K. et al. Progranulin and chemerin plasma level in obese patients with type 2 diabetes treated with a long-acting insulin analogue and premixed insulin analogue. J. Physiol. Pharmacol. 2021; 72(6), doi: 10.26402/jpp.2021.6.07.
42.
Neuparth M.J., Proença J.B., Santos‐Silva A., Coimbra S. The positive effect of moderate walking exercise on chemerin levels in Portuguese patients with type 2 diabetes mellitus. J. Investig. Med. 2014; 62(2): 350–353, doi: 10.2310/JIM.0000000000000025.
43.
Bobbert T., Schwärz F., Fischer-Rosinský A., Maurer L., Möhlig M., Pfeiffer A.F.H. et al. Chemerin and prediction of Diabetes mellitus type 2. Clin. Endocrinol. 2015; 82(6): 838–843, doi: 10.1111/cen.12707.
44.
Tan L., Lu X., Danser A.H.J., Verdonk K. The role of chemerin in metabolic and cardiovascular disease: A literature review of its physiology and pathology from a nutritional perspective. Nutrients 2023; 15(13): 2878, doi: 10.3390/nu15132878.
45.
Mačvanin M.T., Rizzo M., Radovanović J., Sönmez A., Paneni F., Isenović E.R. Role of chemerin in cardiovascular diseases. Biomedicines 2022; 10(11): 2970, doi: 10.3390/biomedicines10112970.
46.
Waluga-Kozłowska E., Komosińska-Vassev K., Szczepański J., Olczyk P. Omentyna – nowy biomarker w medycynie? Część 1. Farm. Pol. 2018; 74(9): 535–541.
47.
Elsaid N.H., Sadik N.A., Ahmed N.R., Fayez S.E., Mohammed N.A.E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J. Clin. Transl. Endocrinol. 2018; 13: 14–19, doi: 10.1016/j.jcte.2018.05.003.
48.
Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C. et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006; 290(6): E1253–1261, doi: 10.1152/ajpendo.00572.2004.
49.
Pan X., Kaminga A.C., Wen S.W., Acheampong K., Liu A. Omentin-1 in diabetes mellitus: A systematic review and meta-analysis. PLoS One 2019; 14(12): e0226292, doi: 10.1371/journal.pone.0226292.
50.
Eimal Latif A.H., Anwar S., Gautham K.S., Kadurei F., Ojo R.O., Hafizyar F. et al. Association of plasma omentin-1 levels with diabetes and its complications. Cureus 2021; 13(9): e18203, doi: 10.7759/cureus.18203.
51.
Biscetti F., Nardella E., Rando M.M., Cecchini A.L., Angelini F., Cina A. et al. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascularization in diabetic patients: a prospective cohort study. Cardiovasc. Diabetol. 2020: 19(1): 170, doi: 10.1186/s12933-020-01151-z.
52.
Askin L., Duman H., Ozyıldız A., Tanrıverdi O., Turkmen S. Association between omentin-1 and coronary artery disease: pathogenesis and clinical research. Curr. Cardiol. Rev. 2020; 16(3): 198–201, doi: 10.2174/1573403X16666200511085304.
CITATIONS (1):
1.
Exploring the role of adipocytokines in obesity and depression
Ruchi Keswani, Vaishnavi G. Thorat, C.R. Patil, Shvetank Bhatt
Drug Discovery Today
Ruchi Keswani, Vaishnavi G. Thorat, C.R. Patil, Shvetank Bhatt
Drug Discovery Today
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.