Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Histopathological analysis of brain lesion samples acquired from stereotactic and navigated brain biopsy: A single cohort study
1
Students’ Scientific Club, Department of Neurosurgery, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
2
Department of Neurosurgery, Faculty of Medical Sciences in Katowice, Medical University of Silesia in Katowice, Poland
3
Department of Pediatric Surgery, Upper Silesian Children’s Health Center named after St. John Paul II, Independent Public Clinical Hospital No. 6, Medical University of Silesia, Katowice, Poland
Corresponding author
Igor Andjelić
Klinika Neurochirurgii, Uniwersyteckie Centrum Kliniczne im. prof. K. Gibińskiego ŚUM, ul. Medyków 14, 40-752 Katowice
Klinika Neurochirurgii, Uniwersyteckie Centrum Kliniczne im. prof. K. Gibińskiego ŚUM, ul. Medyków 14, 40-752 Katowice
Ann. Acad. Med. Siles. 2026;80:51-58
KEYWORDS
TOPICS
ABSTRACT
Introduction:
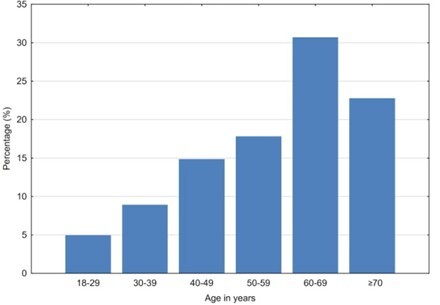
Brain tumors are a complex group of neoplasms originating from various cell types within the central nervous system. Their classification, based on histopathological and molecular characteristics, guides therapeutic strategies and prognosis. Advances in neuroimaging and biopsy techniques have enhanced diagnostic accuracy, allowing for tailored and more effective treatment approaches. Brain biopsy (BB) is an indispensable neurosurgical procedure for the histological diagnosis of neoplastic brain lesions, playing a pivotal role in patient management. The aim of this study is to evaluate the histological outcomes of BBs and to identify the age groups, gender distribution, topography, and different histological types of brain tumors.
Material and methods:
We conducted a retrospective cohort study at a single academic medical center, analyzing 112 patients who underwent BB between January 2017 and August 2023. The study focused on the histological results and molecular markers obtained from stereotactic and neuronavigated brain biopsies, examining the success rate in achieving diagnostic samples and the application of the brain tumor classification system developed by the World Health Organization (WHO). We also studied correlations between histological result and complications.
Results:
Histological examination confirmed the diagnostic accuracy of BB, with a similar distribution of success between stereotactic and neuronavigated methods. The WHO classification of brain tumors was applied to categorize the lesions, which facilitated standardized treatment planning. The study observed a low rate of complications, demonstrating the procedure’s safety.
Conclusions:
The findings of this study indicate that the spectrum of brain tumor diagnoses in our cohort closely parallels global epidemiological trends. The WHO classification framework enabled enhanced diagnostic precision and facilitated standardized therapeutic decision-making.
Brain tumors are a complex group of neoplasms originating from various cell types within the central nervous system. Their classification, based on histopathological and molecular characteristics, guides therapeutic strategies and prognosis. Advances in neuroimaging and biopsy techniques have enhanced diagnostic accuracy, allowing for tailored and more effective treatment approaches. Brain biopsy (BB) is an indispensable neurosurgical procedure for the histological diagnosis of neoplastic brain lesions, playing a pivotal role in patient management. The aim of this study is to evaluate the histological outcomes of BBs and to identify the age groups, gender distribution, topography, and different histological types of brain tumors.
Material and methods:
We conducted a retrospective cohort study at a single academic medical center, analyzing 112 patients who underwent BB between January 2017 and August 2023. The study focused on the histological results and molecular markers obtained from stereotactic and neuronavigated brain biopsies, examining the success rate in achieving diagnostic samples and the application of the brain tumor classification system developed by the World Health Organization (WHO). We also studied correlations between histological result and complications.
Results:
Histological examination confirmed the diagnostic accuracy of BB, with a similar distribution of success between stereotactic and neuronavigated methods. The WHO classification of brain tumors was applied to categorize the lesions, which facilitated standardized treatment planning. The study observed a low rate of complications, demonstrating the procedure’s safety.
Conclusions:
The findings of this study indicate that the spectrum of brain tumor diagnoses in our cohort closely parallels global epidemiological trends. The WHO classification framework enabled enhanced diagnostic precision and facilitated standardized therapeutic decision-making.
REFERENCES (28)
1.
Ostrom QT, Francis SS, Barnholtz-Sloan JS. Epidemiology of Brain and Other CNS Tumors. Curr Neurol Neurosci Rep. 2021;21(12):68. doi: 10.1007/s11910-021-01152-9.
2.
Scheithauer BW. Development of the WHO Classification of Tumors of the Central Nervous System: A Historical Perspective. Brain Pathol. 2008;19(4):551–564. doi: 10.1111/j.1750-3639.2008.00192.x.
3.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1.
4.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106.
5.
Hardigan AA, Jackson JD, Patel AP. Surgical Management and Advances in the Treatment of Glioma. Semin Neurol. 2023;43(6):810–824. doi: 10.1055/s-0043-1776766.
6.
Ma R, Taphoorn MJB, Plaha P. Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry. 2021;92(10):1103–1111. doi: 10.1136/jnnp-2020-325334.
7.
Rodríguez-Camacho A, Flores-Vázquez JG, Moscardini-Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce CS, et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int J Mol Sci. 2022;23(13):7207. doi: 10.3390/ijms23137207.
8.
Lee JH, Wee CW. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabil. 2022;15(3):e24. doi: 10.12786/bn.2022.15.e24.
9.
Jia JL, Alshamsan B, Ng TL. Temozolomide Chronotherapy in Glioma: A Systematic Review. Curr Oncol. 2023;30(2):1893–1902. doi: 10.3390/curroncol30020147.
10.
Babaloui S, Najafi M, Mozdarani H, Borhani S, Jaberi R, Aghili M. Radiosensitization of Glioma Cells by Temozolomide (TMZ): A Colony Formation Assay. J Biomed Phys Eng. 2022;12(1):43–50. doi: 10.31661/jbpe.v0i0.1223.
11.
Kaina B, Beltzig L, Strik H. Temozolomide - Just a Radiosensitizer? Front Oncol. 2022;12:912821. doi: 10.3389/fonc.2022.912821.
12.
Curry RC, Dahiya S, Alva Venur V, Raizer JJ, Ahluwalia MS. Bevacizumab in high-grade gliomas: past, present, and future. Expert Rev Anticancer Ther. 2015;15(4):387–397. doi: 10.1586/14737140.2015.1028376.
13.
Tamura R, Tanaka T, Miyake K, Yoshida K, Sasaki H. Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017;34(2):62–77. doi: 10.1007/s10014-017-0284-x.
14.
Santos M, Roque R, Rainha Campos A, Albuquerque L, Pimentel J. Impact of brain biopsy on management of nonneoplastic brain disease. Brain Spine. 2022;2:100863. doi: 10.1016/j.bas.2022.100863.
15.
Jensen RL, Stone JL, Hayne RA. Introduction of the human Horsley–Clarke stereotactic frame. Neurosurgery. 1996;38(3):563–567; discussion 567. doi: 10.1097/00006123-199603000-00029.
16.
Rahman M, Murad GJA, Mocco J. Early history of the stereotactic apparatus in neurosurgery. Neurosurg Focus. 2009;27(3):E12. doi: 10.3171/2009.7.FOCUS09118.
17.
Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 2012;9(5):491–500. doi: 10.1586/erd.12.42.
18.
Di Bonaventura R, Montano N, Giordano M, Gessi M, Gaudino S, Izzo A, et al. Reassessing the Role of Brain Tumor Biopsy in the Era of Advanced Surgical, Molecular, and Imaging Techniques–A Single-Center Experience with Long-Term Follow-Up. J Pers Med. 2021;11(9):909. doi: 10.3390/jpm11090909.
19.
Ivanov M, Ciurea AV. Neuronavigation. Principles. Surgical technique. J Med Life. 2009;2(1):29–35.
20.
Setyawan NH, Choridah L, Nugroho HA, Malueka RG, Dwianingsih EK. Beyond invasive biopsies: using VASARI MRI features to predict grade and molecular parameters in gliomas. Cancer Imaging. 2024;24(1):3. doi: 10.1186/s40644-023-00638-8.
21.
Chen J, Cen B, Hu F, Qiu Y, Xiao G, Zhou J, et al. Primary Brainstem Lymphoma: A Population-Based Study. Front Surg. 2022;9:829048. doi: 10.3389/fsurg.2022.829048.
22.
Trebouet A, Marchand T, Lemal R, Gyan E, Broussais-Guillaumot F, Guillermin Y, et al. Lymphoma occurring in patients over 90 years of age: characteristics, outcomes, and prognostic factors. A retrospective analysis of 234 cases from the LYSA. Ann Oncol. 2013;24(10):2612–2618. doi: 10.1093/annonc/mdt282.
23.
Choday S, Tran E, Gonzalez M. Diffuse large B-cell lymphoma: examining evolving patterns in mortality, incidence, and demographics. Clin Transl Oncol. 2025;27(8):3432–3438. doi: 10.1007/s12094-025-03859-4.
24.
Lopez Arini M. Diffuse Large B-Cell Lymphoma (DLBCL) / Epidemiology. Rare Disease Advisor [online] https://www.rarediseaseadvi-so... [accessed on 6 January 2024].
25.
Anderson MD, Gilbert MR. Treatment recommendations for anaplastic oligodendrogliomas that are codeleted. Oncology (Williston Park). 2013;27(4):315–320, 322.
26.
Ahmad H, Schiff D. Diffuse Gliomas (WHO Grade 2–3). In: Neuro-Oncology Compendium for the Boards and Clinical Practice. M.M. Mrugala, N.T. Gatson, J.L. Clarke, S.C. Kurz, K.S. Nevel. Oxford University Press; New York, 2023 [online edn]; p. 76–86. doi: 10.1093/med/9780197573778.003.0004.
27.
Kapoor M, Mukkamalla SKR, Gupta V. Astrocytoma. [Updated 2024 May 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-; https://www.ncbi.nlm.nih.gov/b....
28.
Giannini C, Scheithauer BW, Burger PC, Brat DJ, Wollan PC, Lach B, et al. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85(9):2033–2045.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.