Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Comparative cytotoxicity of perphenazine on different human glioblastoma cells
1
Department of Drug and Cosmetics Technology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Center for Natural and Human Sciences, Federal University of ABC, Santo André, São Paulo State, Brazil
3
Department of Toxicology, Toxicological Analysis and Bioanalysis, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Corresponding author
Michał Otręba
Zakład Technologii Środków Leczniczych i Kosmetycznych, Wydział Nauk Farmaceutycznych w Sosnowcu, ul. Jedności 8, 41-200 Sosnowiec
Zakład Technologii Środków Leczniczych i Kosmetycznych, Wydział Nauk Farmaceutycznych w Sosnowcu, ul. Jedności 8, 41-200 Sosnowiec
Ann. Acad. Med. Siles. 2025;79:206-212
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Despite medical advances glioblastoma multiforme (GBM) is still the most common malignant primary brain tumor. Additionally, the gold standard treatment possesses poor (only 12–15 months) survival median. Thus, drug repurposing may be a helpful strategy for discovering more effective GBM chemotherapeutic drugs. Interestingly, phenothiazine derivatives have been considered a promising candidate for drug repurposing for cancer therapy, since they possess several biological activities, such as anticancer, antibacterial, antifungal, and antiviral effects.
Material and methods:
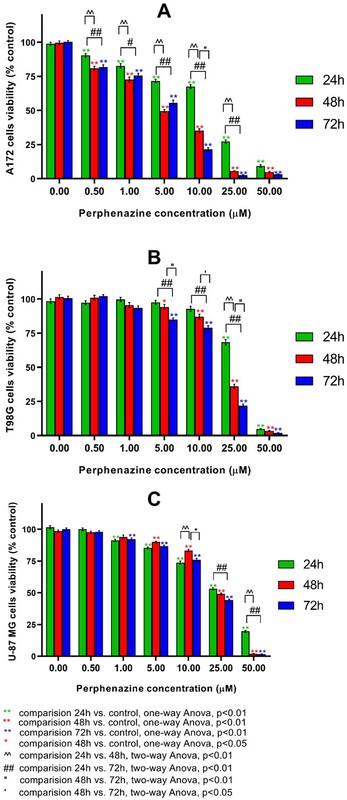
We investigated the impact of perphenazine on the viability of several human glioblastoma (U-87 MG, A172, and T98G) cell lines after 24-, 48-, and 72-hour incubation using WST-1 assay.
Results:
Data showed that the tested phenothiazine derivative decrease glioblastoma viability in a time- and concentration-dependent manner.
Conclusions:
Based on EC50 values, perphenazine is the most efficient against A172 human glioblastoma in all of the tested treatment time periods compared to T98G and U-87 MG cells. Based on previous research, which revealed that perphenazine does not affect normal human astrocytes, this drug is a promising candidate for glioblastoma treatment. Further studies are required to unravel the complete antitumor mechanism of these phenothiazine derivatives in GBM.
Despite medical advances glioblastoma multiforme (GBM) is still the most common malignant primary brain tumor. Additionally, the gold standard treatment possesses poor (only 12–15 months) survival median. Thus, drug repurposing may be a helpful strategy for discovering more effective GBM chemotherapeutic drugs. Interestingly, phenothiazine derivatives have been considered a promising candidate for drug repurposing for cancer therapy, since they possess several biological activities, such as anticancer, antibacterial, antifungal, and antiviral effects.
Material and methods:
We investigated the impact of perphenazine on the viability of several human glioblastoma (U-87 MG, A172, and T98G) cell lines after 24-, 48-, and 72-hour incubation using WST-1 assay.
Results:
Data showed that the tested phenothiazine derivative decrease glioblastoma viability in a time- and concentration-dependent manner.
Conclusions:
Based on EC50 values, perphenazine is the most efficient against A172 human glioblastoma in all of the tested treatment time periods compared to T98G and U-87 MG cells. Based on previous research, which revealed that perphenazine does not affect normal human astrocytes, this drug is a promising candidate for glioblastoma treatment. Further studies are required to unravel the complete antitumor mechanism of these phenothiazine derivatives in GBM.
FUNDING
This study was funded by the Medical University of Silesia, Katowice, Poland (Grant number PCN-2-019/K/3/F).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
REFERENCES (18)
1.
Lan Z., Li X., Zhang X. Glioblastoma: An update in pathology, molecular mechanisms and biomarkers. Int. J. Mol. Sci. 2024; 25(5): 3040, doi: 10.3390/ijms25053040.
2.
Astrocytoma: Grade 4 – Glioblastoma (GBM). National Brain Tumor Society [online] https://braintumor.org/events/... [accessed on 9 July 2024].
3.
Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J. Clin. 2024; 74(1): 12–49, doi: 10.3322/caac.21820.
4.
Brain CNS [pdf]. Global Cancer Observatory: Cancer Today / International Agency for Research on Cancer [online] https://gco.iarc.who.int/media... [accessed on 9 July 2024].
5.
Lopes R.M., Souza A.C.S., Otręba M., Rzepecka-Stojko A., Tersariol I.L.S., Rodrigues T. Targeting autophagy by antipsychotic phenothiazines: potential drug repurposing for cancer therapy. Biochem. Pharmacol. 2024; 222: 116075, doi: 10.1016/j.bcp.2024.116075.
6.
Cerner Multum. Perphenazine. Drugs.com: Know more. Be sure, Aug 3, 2023 [online] https://www.drugs.com/mtm/perp... [accessed on 9 July 2024].
7.
Edinoff A.N., Armistead G., Rosa C.A., Anderson A., Patil R., Cornett E.M. et al. Phenothiazines and their evolving roles in clinical practice: A narrative review. Health Psychol. Res. 2022; 10(4): 38930, doi: 10.52965/001c.38930.
8.
Jeleń M., Morak-Młodawska B., Korlacki R. Anticancer activities of tetra-, penta-, and hexacyclic phenothiazines modified with quinoline moiety. J. Mol. Struct. 2023; 1287: 135700, doi: 10.1016/j.molstruc.2023.135700.
9.
Otręba M., Kośmider L., Rzepecka-Stojko A. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 2020; 887: 173553, doi: 10.1016/j.ejphar.2020.173553.
10.
Otręba M., Kośmider L. In vitro anticancer activity of fluphenazine, perphenazine and prochlorperazine. A review. J. Appl. Toxicol. 2021; 41(1): 82–94, doi: 10.1002/jat.4046.
11.
Mello J.C., Moraes V.W., Watashi C.M., da Silva D.C., Cavalcanti L.P., Franco M.K. et al. Enhancement of chlorpromazine antitumor activity by Pluronics F127/L81 nanostructured system against human multidrug resistant leukemia. Pharmacol. Res. 2016; 111: 102–112, doi: 10.1016/j.phrs.2016.05.032.
12.
Otręba M., Wrześniok D., Rok J., Beberok A., Buszman E. Prochlorperazine interaction with melanin and melanocytes. Pharmazie 2017; 72(3): 171–176, doi: 10.1691/ph.2017.6787.
13.
Gil-Ad I., Shtaif B., Levkovitz Y., Dayag M., Zeldich E., Weizman A. Characterization of phenothiazine-induced apoptosis in neuroblastoma and glioma cell lines: clinical relevance and possible application for brain-derived tumors. J. Mol. Neurosci. 2004; 22(3): 189–198, doi: 10.1385/JMN:22:3:189.
14.
Tzadok S., Beery E., Israeli M., Uziel O., Lahav M., Fenig E. et al. In vitro novel combinations of psychotropics and anti-cancer modalities in U87 human glioblastoma cells. Int. J. Oncol. 2010; 37(4): 1043–1051, doi: 10.3892/ijo_00000756.
15.
Cheng H.W., Liang Y.H., Kuo Y.L., Chuu C.P., Lin C.Y., Lee M.H. et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015; 6(5): e1753, doi: 10.1038/cddis.2015.77.
16.
Otręba M., Buszman E. Perphenazine and prochlorperazine induce concentration-dependent loss in human glioblastoma cells viability. Pharmazie 2018; 73(1): 19–21, doi: 10.1691/ph.2018.7806.
17.
Jacob J.R., Palanichamy K., Chakravarti A. Antipsychotics possess anti-glioblastoma activity by disrupting lysosomal function and inhibiting oncogenic signaling by stabilizing PTEN. Cell Death Dis. 2024; 15(6): 414, doi: 10.1038/s41419-024-06779-3.
18.
Otręba M., Rzepecka-Stojko A., Stojko J., Kabała-Dzik A., Kleczka A. Effect of perphenazine and prochlorperazine on viability of human astrocytes. Ann. Acad. Med. Siles. 2024; 78: 167–172, doi: 10.18794/aams/177539.
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.