Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Echocardiographic markers of left ventricular hypertrophy and concentric remodeling – limitations in diagnostics of cardiac amyloidosis, Fabry disease and hypertrophic cardiomyopathy
1
1st Department of Cardiology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Corresponding author
Mateusz Tometczak
Klinika Kardiologii I Katedry Kardiologii, Górnośląskie Centrum Medyczne im. prof. Leszka Gieca Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Ziołowa 47, 40-635 Katowice
Klinika Kardiologii I Katedry Kardiologii, Górnośląskie Centrum Medyczne im. prof. Leszka Gieca Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Ziołowa 47, 40-635 Katowice
Ann. Acad. Med. Siles. 2024;78:219-225
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Left ventricular hypertrophy (LVH) is a relevant sign associated with an increased risk of sudden death. The causes of LVH including cardiac amyloidosis (CA), Fabry disease (FD), hypertrophic cardiomyopathy (HCM) are associated with an inauspicious prognosis. Transthoracic echocardiography (TTE) remains the first-step baseline diagnostic method.
Material and methods:
A retrospective one-center analysis of 86 patients (pts) with increased left ventricular (LV) wall thickness in TTE was performed. The inclusion criteria were interventricular septum (IVS) above 10 mm in males, 9 mm in females and the final diagnosis of CA, FD or HCM. The study population was divided into three subgroups: CA (13 pts), FD (7 pts), HCM (66 pts). The LV mass index (LVMI), relative wall thickness (RWT) and type of remodeling were analyzed.
Results:
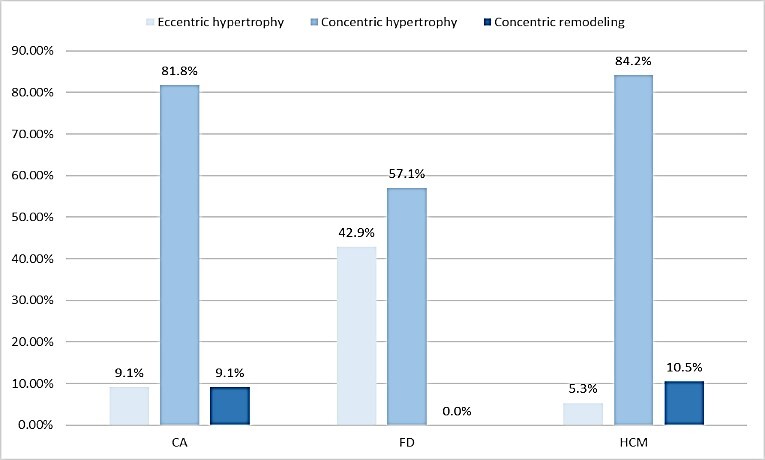
Increased LVMI occurred in 90.9% pts with CA, all with FD, 89.5% with HCM.RWT exceeded the normal range among 92.3% pts with CA, 57.1% with FD, 92.4% with HCM. Concentric hypertrophy was diagnosed in 75% pts with CA, 57.1% with FD, 84.2% with HCM and eccentric in 8.3% pts with CA, 42.9% with FD, 5.3% with HCM (p = 0.01). An abnormal IVS/PWT index was observed in 23.1% pts with CA, 28.6% with FD, 79.7% with HCM (p = 0.00001).
Conclusions:
Although cardiac hypertrophy is a typical sign, it does not occur in all subjects with CA, FD, HCM. More detailed analysis including the form of hypertrophy as well as left atrium remodeling are required to be characterized for specific diseases: CA, FD, HCM. Asymmetrical hypertrophy is more specific for HCM.
Left ventricular hypertrophy (LVH) is a relevant sign associated with an increased risk of sudden death. The causes of LVH including cardiac amyloidosis (CA), Fabry disease (FD), hypertrophic cardiomyopathy (HCM) are associated with an inauspicious prognosis. Transthoracic echocardiography (TTE) remains the first-step baseline diagnostic method.
Material and methods:
A retrospective one-center analysis of 86 patients (pts) with increased left ventricular (LV) wall thickness in TTE was performed. The inclusion criteria were interventricular septum (IVS) above 10 mm in males, 9 mm in females and the final diagnosis of CA, FD or HCM. The study population was divided into three subgroups: CA (13 pts), FD (7 pts), HCM (66 pts). The LV mass index (LVMI), relative wall thickness (RWT) and type of remodeling were analyzed.
Results:
Increased LVMI occurred in 90.9% pts with CA, all with FD, 89.5% with HCM.RWT exceeded the normal range among 92.3% pts with CA, 57.1% with FD, 92.4% with HCM. Concentric hypertrophy was diagnosed in 75% pts with CA, 57.1% with FD, 84.2% with HCM and eccentric in 8.3% pts with CA, 42.9% with FD, 5.3% with HCM (p = 0.01). An abnormal IVS/PWT index was observed in 23.1% pts with CA, 28.6% with FD, 79.7% with HCM (p = 0.00001).
Conclusions:
Although cardiac hypertrophy is a typical sign, it does not occur in all subjects with CA, FD, HCM. More detailed analysis including the form of hypertrophy as well as left atrium remodeling are required to be characterized for specific diseases: CA, FD, HCM. Asymmetrical hypertrophy is more specific for HCM.
REFERENCES (20)
1.
Melero Polo J., Roteta Unceta-Barrenechea A., Revilla Martí P., Pérez-Palacios R., Gracia Gutiérrez A., Bueno Juana E. et al. Echocardiographic markers of cardiac amyloidosis in patients with heart failure and left ventricular hypertrophy. Cardiol. J. 2023; 30(2): 266–275, doi: 10.5603/CJ.a2021.0085.
2.
Tanaka H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: special focus on speckle-tracking longitudinal strain. J. Echocardiogr. 2021; 19(2): 71–79, doi: 10.1007/s12574-020-00508-3.
3.
Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015; 16(3): 233–270, doi: 10.1093/ehjci/jev014. Erratum in: Eur. Heart J. Cardiovasc. Imaging 2016; 17(4): 412. Erratum in: Eur. Heart J. Cardiovasc. Imaging 2016; 17(9): 969.
4.
Bula K., Ćmiel A., Sejud M., Sobczyk K., Ryszkiewicz S., Szydło K. et al. Electrocardiographic criteria for left ventricular hypertrophy in aortic valve stenosis: Correlation with echocardiographic parameters. Ann. Noninvasive Electrocardiol. 2019; 24(5): e12645, doi: 10.1111/anec.12645.
5.
Wang S., Song K., Guo X., Xue H., Wang N., Chen J. et al. The association of metabolic syndrome with left ventricular mass and geometry in community-based hypertensive patients among Han Chinese. J. Res. Med. Sci. 2015; 20(10): 963–968, doi: 10.4103/1735-1995.172785.
6.
Chahal N.S., Lim T.K., Jain P., Chambers J.C., Kooner J.S., Senior R. New insights into the relationship of left ventricular geometry and left ventricular mass with cardiac function: A population study of hypertensive subjects. Eur. Heart J. 2010; 31(5): 588–594, doi: 10.1093/eurheartj/ehp490.
7.
Seko Y., Kato T., Haruna T., Izumi T., Miyamoto S., Nakane E. et al. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci. Rep. 2018; 8(1): 6366, doi: 10.1038/s41598-018-24875-1.
8.
Stewart M.H., Lavie C.J., Shah S., Englert J., Gilliland Y., Qamruddin S. et al. Prognostic implications of left ventricular hypertrophy. Prog. Cardiovasc. Dis. 2018; 61(5–6): 446–455, doi: 10.1016/j.pcad.2018.11.002.
9.
Cardim N., Galderisi M., Edvardsen T., Plein S., Popescu B.A., D’Andrea A. et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur. Heart J. Cardiovasc. Imaging 2015; 16(3): 280, doi: 10.1093/ehjci/jeu291.
10.
Maceira A.M., Cosín-Sales J., Roughton M., Prasad S.K., Pennell D.J. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010; 12(1): 65, doi: 10.1186/1532-429X-12-65.
11.
Perry R., Selvanayagam J.B. Echocardiography in infiltrative cardiomyopathy. Heart Lung Circ. 2019; 28(9): 1365–1375, doi: 10.1016/j.hlc.2019.04.017.
12.
Nagueh S.F., Phelan D., Abraham T., Armour A., Desai M.Y., Dragulescu A. et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: An update from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2022; 35(6): 533–569, doi: 10.1016/j.echo.2022.03.012.
13.
Saeed S., Imazio M. Fabry disease: definition, incidence, clinical presentations and treatment – focus on cardiac involvement. Pak. J. Med. Sci. 2022; 38(8): 2337–2344, doi: 10.12669/pjms.38.8.7063.
14.
Yuasa T., Takenaka T., Higuchi K., Uchiyama N., Horizoe Y., Cyaen H. et al. Fabry disease. J. Echocardiogr. 2017; 15(4): 151–157, doi: 10.1007/s12574-017-0340-x.
15.
Patil P.V., Wiegers S.E. Echocardiography for hypertrophic cardiomyopathy. Prog. Cardiovasc. Dis. 2014; 57(1): 91–99, doi: 10.1016/j.pcad.2014.05.007.
16.
Haland T.F., Edvardsen T. The role of echocardiography in management of hypertrophic cardiomyopathy. J. Echocardiogr. 2020; 18(2): 77–85, doi: 10.1007/s12574-019-00454-9.
17.
Stankowski K., Figliozzi S., Battaglia V., Catapano F., Francone M., Monti L. Fabry disease: more than a phenocopy of hypertrophic cardiomyopathy. J. Clin. Med. 2023; 12(22): 7061, doi: 10.3390/jcm12227061.
18.
Liang S., Liu Z., Li Q., He W., Huang H. Advance of echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2023; 28(6): 1345–1356, doi: 10.1007/s10741-023-10332-3.
19.
Falk R.H., Quarta C.C. Echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2015; 20(2): 125–131, doi: 10.1007/s10741-014-9466-3.
20.
Dominguez F., González-López E., Padron-Barthe L., Cavero M.A., Garcia-Pavia P. Role of echocardiography in the diagnosis and management of hypertrophic cardiomyopathy. Heart 2018; 104(3): 261–273, doi: 10.1136/heartjnl-2016-310559.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.