Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Evaluation of photoprotective properties of pharmaceutical packaging containing cefuroxime by total hemispherical reflectance analysis
1
Department of Basic Biomedical Sciences, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Doctoral School, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Corresponding author
Michał Meisner
Katedra i Zakład Podstawowych Nauk Biomedycznych, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-200 Sosnowiec
Katedra i Zakład Podstawowych Nauk Biomedycznych, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-200 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:226-233
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Improper protection of an active substance may lead to a loss of its original properties. Many methods can be used to assess the photoprotective properties of drug packaging, including the hemispherical directional reflectance method. The aim of the study is to assess the reflectance value for both the outer and direct packaging containing cefuroxime.
Material and methods:
Two formulations (Ceroxim and Zinnat) of both expired and unexpired packages were tested with a 410-Solar reflectometer. Three types of measurement areas were analyzed: “white” (white areas of the outer cardboard box), “coloured” (coloured areas of the outer cardboard box) and “blister” (the direct packaging made of aluminium/PVC).
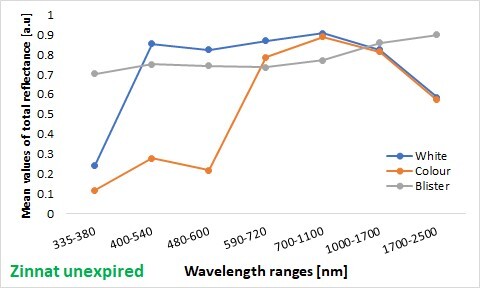
Results:
The highest reflectance value was found in the 700–1100 nm range for both the unexpired and expired preparations. In the 335–380 nm band, the amount of reflected radiation for the unexpired Ceroxim vs Zinnat packages was as follows: 30% vs 12% from the coloured areas, 39% vs 24% from the white areas and 74% vs 70% from the blisters, respectively. For the expired coloured areas of Ceroxim, the reflectance was significantly higher compared to the unexpired (p < 0.001) in all the spectral bands, except 1700–2500 nm. In contrast, the reflectance of the expired white areas of Ceroxim was higher than the unexpired (p < 0.001) for 480–600 nm, 590–720 nm, 700–1100 nm, and 1700–2500 nm. The blisters of the unexpired Zinnat preparation exhibited greater photoprotective properties than the expired in the 335–380 nm range while the unexpired and expired blisters of Ceroxim did not differ.
Conclusions:
Based on the reflectance value, blisters and white cardboard packages protect cefuroxime against radiation to a greater extent than coloured packages.
Improper protection of an active substance may lead to a loss of its original properties. Many methods can be used to assess the photoprotective properties of drug packaging, including the hemispherical directional reflectance method. The aim of the study is to assess the reflectance value for both the outer and direct packaging containing cefuroxime.
Material and methods:
Two formulations (Ceroxim and Zinnat) of both expired and unexpired packages were tested with a 410-Solar reflectometer. Three types of measurement areas were analyzed: “white” (white areas of the outer cardboard box), “coloured” (coloured areas of the outer cardboard box) and “blister” (the direct packaging made of aluminium/PVC).
Results:
The highest reflectance value was found in the 700–1100 nm range for both the unexpired and expired preparations. In the 335–380 nm band, the amount of reflected radiation for the unexpired Ceroxim vs Zinnat packages was as follows: 30% vs 12% from the coloured areas, 39% vs 24% from the white areas and 74% vs 70% from the blisters, respectively. For the expired coloured areas of Ceroxim, the reflectance was significantly higher compared to the unexpired (p < 0.001) in all the spectral bands, except 1700–2500 nm. In contrast, the reflectance of the expired white areas of Ceroxim was higher than the unexpired (p < 0.001) for 480–600 nm, 590–720 nm, 700–1100 nm, and 1700–2500 nm. The blisters of the unexpired Zinnat preparation exhibited greater photoprotective properties than the expired in the 335–380 nm range while the unexpired and expired blisters of Ceroxim did not differ.
Conclusions:
Based on the reflectance value, blisters and white cardboard packages protect cefuroxime against radiation to a greater extent than coloured packages.
FUNDING
The study was funded within the project PCN-1-058/K/2/O by the Medical University of Silesia, Katowice, Poland.
REFERENCES (13)
1.
Waterman K.C., MacDonald B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010; 99(11): 4437–4452, doi: 10.1002/jps.22161.
2.
Poitou P. The role of the packaging in terms of safety and good use of medicines. [Article in French]. Ann. Pharm. Fr. 2003; 61(5): 300–303.
3.
McPherson T., Kolling W.M. Stability and compatibility study guidelines for AJHP. Am. J. Health Syst. Pharm. 2023; 80(18): 1271–1274, doi: 10.1093/ajhp/zxad132.
4.
Berendsen B.J.A., Elbers I.J.W., Stolker A.A.M. Determination of the stability of antibiotics in matrix and reference solutions using a straightforward procedure applying mass spectrometric detection. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011; 28(12): 1657–1666, doi: 10.1080/19440049.2011.604045.
5.
Bhutani H., Mariappan T.T., Singh S. The physical and chemical stability of anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int. J. Tuberc. Lung Dis. 2004; 8(9): 1073–1080.
6.
Lin X., Kück U. Cephalosporins as key lead generation beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2022; 106(24): 8007–8020, doi: 10.1007/s00253-022-12272-8.
7.
Błoński B., Wilczyński S., Hartman-Petrycka M., Michalecki Ł. The use of hemispherical directional reflectance to evaluate the interaction of food products with radiation in the solar spectrum. Foods 2022; 11(13): 1974, doi: 10.3390/foods11131974.
8.
Kundu P., Bandyopadhyay S., Trémeau A. Analysis of spectral differences between printers to detect the counterfeit medicine packaging. J. Algebr. Stat. 2022; 13(2): 798–806.
9.
Çapkın İ.Y., Gökelma M. A review on characterization and recyclability of pharmaceutical blisters. Cleaner Waste Systems 2023; 4: 100082, doi: 10.1016/j.clwas.2023.100082.
10.
Matyukhin P.V. The choice of iron-containing filling for composite radioprotective material. IOP Conf. Ser.: Mater. Sci. Eng. 2018; 327: 032036, doi: 10.1088/1757-899X/327/3/032036.
11.
Li X., Yang J., Qiao Y., Duan Y., Xin Y., Nian Y. et al. Effects of radiation on drug metabolism: A review. Curr. Drug Metab. 2019; 20(5): 350–360, doi: 10.2174/1389200220666190405171303.
12.
McGill M.R., Findley D.L., Mazur A., Yee E.U., Allard F.D., Powers A. et al. Radiation effects on methamphetamine pharmacokinetics and pharmacodynamics in rats. Eur. J. Drug Metab. Pharmacokinet. 2022; 47(3): 319–330, doi: 10.1007/s13318-022-00755-y.
13.
Glass B.D., Novák C., Brown M.E. The thermal and photostability of solid pharmaceuticals. J. Therm. Anal. Calorim. 2004; 77: 1013–1036, doi: 10.1023/B:JTAN.0000041677.48299.25.
CITATIONS (1):
1.
The Impact of UV Radiation on the Hemispherical Reflectance Values and Homogeneity of Tablets Containing Clindamycin and Phenoxymethylpenicillin
Michał Meisner, Beata Sarecka-Hujar
Applied Sciences
Michał Meisner, Beata Sarecka-Hujar
Applied Sciences
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.