Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Tick-borne encephalitis and differential diagnosis
1
Studenckie Koło Naukowe przy Oddziale Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, Polska / Students’ Scientific Club, Department of Pediatrics and Developmental Neurology, Upper Silesian Child Health Center named after John Paul II, Independent Public Clinical Hospital No. 6 of the Medical University of Silesia, Katowice, Poland
2
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, Polska / Department of Pediatrics and Developmental Neurology, Upper Silesian Child Health Center named after John Paul II, Independent Public Clinical Hospital No. 6 of the Medical University of Silesia, Katowice, Poland
Corresponding author
Patrycja Ochman-Pasierbek
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Ann. Acad. Med. Siles. 2024;78:234-247
KEYWORDS
TOPICS
ABSTRACT
Introduction:
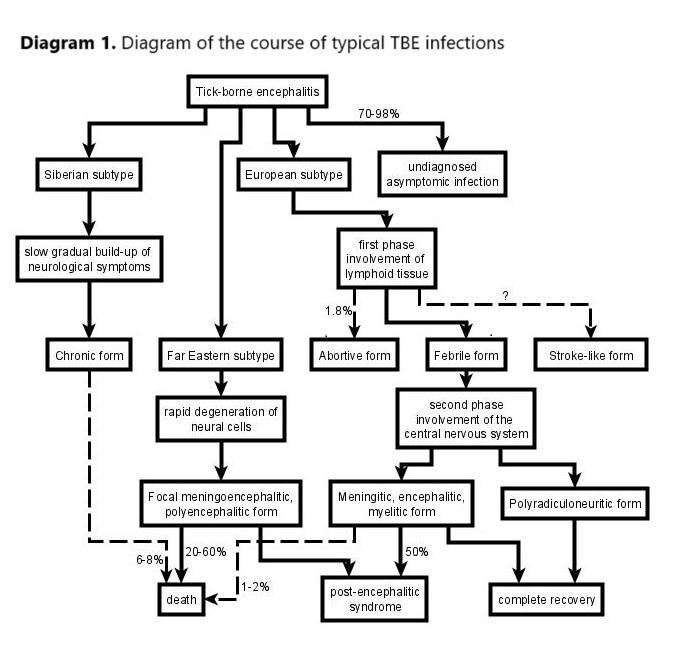
Tick-borne encephalitis (TBE) is an infection caused by the tick-borne encephalitis virus transmitted to humans by tick bites. The prevalence of TBE is between 10,000 and 15,000 cases annually and is comparable in Europe and Asia. About 10–20% of all infected persons are children. The vast majority of TBE cases, even up to 70–98% of them, are asymptomatic or undiagnosed. The main clinical symptoms are meningitis (present in 69%), meningoencephalitis (30%), and meningoencephalomyelitis (1%). About 2.1% of patients develop long-term neurological sequelae.
Methods:
The articles for our work were selected from three open-access databases. The databases were searched using keywords such as: “infection/epidemiology” + “tick bites/tick-borne encephalitis” + “clinical manifestation/pathogenesis/treatment” and “aseptic/viral/bacterial” + “encephalitis/meningitis”. Ultimately, 71 scientific articles and 8 websites, published between 1995 to 2023 were used.
State of knowledge:
TBE can be differentiated from diseases such as babesiosis, Lyme disease, southern tick-associated rash illness (STARI), chlamydiosis, ehrlichiosis, Colorado tick fever (CTF), Heartland virus (HRTV), Powassan virus (POWV), granulocytic anaplasmosis, tick-borne relapsing fever (TBRF), toxoplasmosis, tularemia, rickettsioses; or similar symptomatology: stroke, brucellosis, infectious mononucleosis (IM), yellow fever (YF), Japanese encephalitis (JE), other viral meningitis, encephalitis, spinal cord inflammation and aseptic meningitis.
Conclusions:
The differential diagnosis of TBE is extensive and should include a wide range of central nervous system infections caused by both other infectious agents and non-infectious diseases.
Tick-borne encephalitis (TBE) is an infection caused by the tick-borne encephalitis virus transmitted to humans by tick bites. The prevalence of TBE is between 10,000 and 15,000 cases annually and is comparable in Europe and Asia. About 10–20% of all infected persons are children. The vast majority of TBE cases, even up to 70–98% of them, are asymptomatic or undiagnosed. The main clinical symptoms are meningitis (present in 69%), meningoencephalitis (30%), and meningoencephalomyelitis (1%). About 2.1% of patients develop long-term neurological sequelae.
Methods:
The articles for our work were selected from three open-access databases. The databases were searched using keywords such as: “infection/epidemiology” + “tick bites/tick-borne encephalitis” + “clinical manifestation/pathogenesis/treatment” and “aseptic/viral/bacterial” + “encephalitis/meningitis”. Ultimately, 71 scientific articles and 8 websites, published between 1995 to 2023 were used.
State of knowledge:
TBE can be differentiated from diseases such as babesiosis, Lyme disease, southern tick-associated rash illness (STARI), chlamydiosis, ehrlichiosis, Colorado tick fever (CTF), Heartland virus (HRTV), Powassan virus (POWV), granulocytic anaplasmosis, tick-borne relapsing fever (TBRF), toxoplasmosis, tularemia, rickettsioses; or similar symptomatology: stroke, brucellosis, infectious mononucleosis (IM), yellow fever (YF), Japanese encephalitis (JE), other viral meningitis, encephalitis, spinal cord inflammation and aseptic meningitis.
Conclusions:
The differential diagnosis of TBE is extensive and should include a wide range of central nervous system infections caused by both other infectious agents and non-infectious diseases.
REFERENCES (79)
1.
Bogovic P., Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015; 3(5): 430–441, doi: 10.12998/wjcc.v3.i5.430.
2.
Gritsun T.S., Lashkevich V.A., Gould E.A. Tick-borne encephalitis. Antiviral Res. 2003; 57(1–2): 129–146, doi: 10.1016/S0166-3542(02)00206-1.
3.
Kmieciak W., Ciszewski M., Szewczyk E.M. Tick-borne diseases in Poland: Prevalence and difficulties in diagnostics. [Article in Polish]. Med. Pr. 2016; 67(1): 73–87, doi: 10.13075/mp.5893.00264.
4.
Zajkowska J., Waluk E., Dunaj J., Świerzbińska R., Hordowicz M., Zajkowska O. et al. Assessment of the potential effect of the implementation of serological testing tick-borne encephalitis on the detection of this disease on areas considered as non-endemic in Poland – preliminary report. Przegl. Epidemiol. 2021; 75(4): 515–523, doi: 10.32394/pe.75.48.
5.
Stragapede L., Dinoto A., Cheli M., Manganotti P. Epilepsia partialis continua following a Western variant tick-borne encephalitis. J. Neurovirol. 2018; 24(6): 773–775, doi: 10.1007/s13365-018-0671-z.
6.
Eggers C., Burghaus L., Fink G.R., Dohmen C. Epilepsia partialis continua responsive to intravenous levetiracetam. Seizure 2009; 18(10): 716–718, doi: 10.1016/j.seizure.2009.09.005.
7.
Mameniškienė R., Wolf P. Epilepsia partialis continua: A review. Seizure 2017; 44: 74–80, doi: 10.1016/j.seizure.2016.10.010.
8.
Motika P.V., Bergen D.C. Epilepsia Partialis Continua. In: Encyclopedia of Movement Disorders. K. Kompoliti, L.V. Metman [ed.]. Academic Press, 2010, p. 450–452, doi: 10.1016/B978-0-12-374105-9.00028-9.
9.
Gritsun T.S., Nuttall P.A., Gould E.A. Tick-borne flaviviruses. Adv. Virus Res. 2003; 61: 317–371, doi: 10.1016/s0065-3527(03)61008-0.
10.
Beltz L.A. Zika and Other Neglected and Emerging Flaviviruses: The Continuing Threat to Human Health. Elsevier 2021.
11.
Růžek D., Dobler G., Mantke O.D. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010; 8(4): 223–232, doi: 10.1016/j.tmaid.2010.06.004.
12.
Eyer L., Seley-Radtke K., Ruzek D. New directions in the experimental therapy of tick-borne encephalitis. Antiviral Res. 2023; 210: 105504, doi: 10.1016/j.antiviral.2022.105504.
13.
Rostasy K. Tick-borne encephalitis in children. Wien. Med. Wochenschr. 2012; 162(11–12): 244–247, doi: 10.1007/s10354-012-0101-4.
14.
Pancewicz S.A., Hermanowska-Szpakowicz T., Kondrusik M., Zajkowska J.M., Grygorczuk S., Świerzbińska R. Aspekty epidemiologiczno-kliniczne i profilaktyka kleszczowego zapalenia mózgu. Pol. Przegl. Neurol. 2006; 2(1): 7–12.
15.
Ruzek D., Avšič Županc T., Borde J., Chrdle A., Eyer L., Karganova G. et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 2019; 164: 23–51, doi: 10.1016/j.antiviral.2019.01.014.
16.
Lindquist L., Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371(9627): 1861–1871, doi: 10.1016/S0140-6736(08)60800-4.
17.
Riccardi N., Antonello R.M., Luzzati R., Zajkowska J., Di Bella S., Giacobbe D.R. Tick-borne encephalitis in Europe: a brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019; 62: 1–6, doi: 10.1016/j.ejim.2019.01.004.
18.
Pulkkinen L.I.A., Butcher S.J., Anastasina M. Tick-borne encephalitis virus: A structural view. Viruses 2018; 10(7): 350, doi: 10.3390/v10070350.
19.
Beauté J., Spiteri G., Warns-Petit E., Zeller H. Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill. 2018; 23(45): 1800201, doi: 10.2807/1560-7917.ES.2018.23.45.1800201.
20.
Amicizia D., Domnich A., Panatto D., Lai P.L., Cristina M.L., Avio U. et al. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccin. Immunother. 2013; 9(5): 1163–1171, doi: 10.4161/hv.23802.
21.
Kuchar E., Zajkowska J., Flisiak R., Mastalerz-Migas A., Rosińska M., Szenborn L. et al. Epidemiology, diagnosis, and prevention of tick-borne encephalitis in Poland and selected European countries – a position statement of the Polish group of experts. [Article in Polish]. Med. Pr. 2021; 72(2): 193–210, doi: 10.13075/mp.5893.01063.
22.
European Centre for Disease Prevention and Control. Tick-borne encephalitis. In: ECDC. Annual epidemiological report for 2020. Stockholm: ECDC; 2022.
23.
Sapi E., Gupta K., Wawrzeniak K., Gaur G., Torres J., Filush K. et al. Borrelia and Chlamydia can form mixed biofilms in infected human skin tissues. Eur. J. Microbiol. Immunol. (Bp) 2019; 9(2): 46–55, doi: 10.1556/1886.2019.00003.
24.
Davar K., Wilson M.R., Miller S., Chiu C.Y., Vijayan T. A rare bird: Diagnosis of psittacosis meningitis by clinical metagenomic next-generation sequencing. Open Forum Infect. Dis. 2021; 8(12): ofab555; doi: 10.1093/ofid/ofab555.
25.
Dard C., Fricker-Hidalgo H., Brenier-Pinchart M.P., Pelloux H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 2016; 32(6): 492–506, doi: 10.1016/j.pt.2016.04.001.
26.
Ben-Harari R.R. Tick transmission of toxoplasmosis. Expert Rev. Anti Infect. Ther. 2019; 17(11): 911–917, doi: 10.1080/14787210.2019.1682550.
27.
Rawlings J.A. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex. Med. 1995; 91(5): 56–59.
28.
Furtado J.M., Smith J.R., Belfort R. Jr, Gattey D., Winthrop K.L. Toxoplasmosis: a global threat. J. Glob. Infect. Dis. 2011; 3(3): 281–284, doi: 10.4103/0974-777X.83536.
29.
Weiss L.M., Dubey J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009; 39(8): 895–901, doi: 10.1016/j.ijpara.2009.02.004.
30.
Elsheikha H.M., Marra C.M., Zhu X.Q. Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin. Microbiol. Rev. 2020; 34(1): e00115–00119, doi: 10.1128/CMR.00115-19.
31.
Fuglewicz A.J., Piotrowski P., Stodolak A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017; 26(6): 1031–1036, doi: 10.17219/acem/61435.
32.
Schoen R.T. Lyme disease: diagnosis and treatment. Curr. Opin. Rheumatol. 2020; 32(3): 247–254, doi: 10.1097/BOR.0000000000000698.
33.
Abdad M.Y., Abou Abdallah R., Fournier P.E., Stenos J., Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018; 56(8): e01728-17, doi: 10.1128/JCM.01728-17.
34.
Jorge Miranda R., Salim Mattar V., Marco Gonzalez T. Rickettsiosis. Rev. MVZ Cordoba 2017; 22(Supl): 6118–6133, doi: 10.21897/rmvz.1080.
35.
Corrin T., Greig J., Harding S., Young I., Mascarenhas M., Waddell L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018; 65(6): 595–624, doi: 10.1111/zph.12485.
36.
Bakken J.S., Dumler J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 2015; 29(2): 341–355, doi: 10.1016/j.idc.2015.02.007.
37.
Factsheet on Human granulocytic anaplasmosis. European Centre for Disease Prevention and Control [online] https://web.archive.org/web/20... [accessed on 6 June 2022].
38.
Cho J.M., Chang J., Kim D.M., Kwak Y.G., Cho C.R., Song J.E. Human granulocytic anaplasmosis combined with rhabdomyolysis: a case report. BMC Infect. Dis. 2021; 21(1): 1184, doi: 10.1186/s12879-021-06869-z.
39.
Kandhi S., Ghazanfar H., Qureshi Z.A., Kalangi H., Jyala A., Arguello Perez E.S. An atypical presentation of a severe case of anaplasma phagocytophilum. Cureus 2022; 14(3): e23224, doi: 10.7759/cureus.23224.
40.
de Jesus M., Lopez A., Yabut J., Vu S., Manne M., Ibrahim L., Mutneja R. Anaplasmosis-induced hemophagocytic lymphohistiocytosis. Proc. (Bayl. Univ. Med. Cent.) 2022; 35(3): 379–381, doi: 10.1080/08998280.2022.2039046.
41.
Abdelmaseih R., Ashraf B., Abdelmasih R., Dunn S., Nasser H. Southern tick-associated rash illness: Florida’s Lyme disease variant. Cureus 2021; 13(5): e15306, doi: 10.7759/cureus.15306.
42.
Nualnoi T., Kirosingh A., Basallo K., Hau D., Gates-Hollingsworth M.A., Thorkildson P. et al. Immunoglobulin G subclass switching impacts sensitivity of an immunoassay targeting Francisella tularensis lipopolysaccharide. PLoS One 2018; 13(4): e0195308, doi: 10.1371/journal.pone.0195308.
43.
Faber M., Heuner K., Jacob D., Grunow R. Tularemia in Germany – A re-emerging zoonosis. Front. Cell. Infect. Microbiol. 2018; 8: 40, doi: 10.3389/fcimb.2018.00040.
44.
Rochlin I., Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 2020; 69(6): 781–791, doi: 10.1099/jmm.0.001206.
45.
Biggs H.M., Behravesh C.B., Bradley K.K., Dahlgren F.S., Drexler N.A., Dumler J.S, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis – United States. MMWR Recomm. Rep. 2016; 65(2): 1–44, doi: 10.15585/mmwr.rr6502a1.
46.
Heartland Virus. Columbia University Irving Medical Center [online] https://www.columbia-lyme.org/... [accessed on 6 June 2022].
47.
Staples J.E., Pastula D.M., Panella A.J., Rabe I.B., Kosoy O.I., Walker W.L. et al. Investigation of Heartland virus disease throughout the United States, 2013–2017. Open Forum Infect. Dis. 2020; 7(5): ofaa125, doi: 10.1093/ofid/ofaa125.
48.
Brault A.C., Savage H.M., Duggal N.K., Eisen R.J., Staples J.E. Heartland virus epidemiology, vector association, and disease potential. Viruses 2018; 10(9): 498, doi: 10.3390/v10090498.
49.
Tuten H.C., Burkhalter K.L., Noel K.R., Hernandez E.J., Yates S., Wojnowski K. et al. Heartland virus in humans and ticks, Illinois, USA, 2018–2019. Emerg. Infect. Dis. 2020; 26(7): 1548–1552, doi: 10.3201/eid2607.200110.
50.
Pastula D.M., Turabelidze G., Yates K.F., Jones T.F., Lambert A.J., Panella A.J. et al. Notes from the field: Heartland virus disease – United States, 2012–2013. MMWR Morb. Mortal. Wkly Rep. 2014; 63(12): 270–271.
51.
Jakab Á., Kahlig P., Kuenzli E., Neumayr A. Tick borne relapsing fever – a systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 2022; 16(2): e0010212, doi: 10.1371/journal.pntd.0010212.
52.
Domínguez M.C., Vergara S., Gómez M.C., Roldán M.E. Epidemiology of tick-borne relapsing fever in endemic area, Spain. Emerg. Infect. Dis. 2020; 26(5): 849–856, doi: 10.3201/eid2605.190745.
53.
Tick and Louse-borne Relapsing Fevers. CDC [online] https://www.cdc.gov/relapsing-... [accessed on 6 June 2022].
55.
Ebel G.D. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol. 2010; 55: 95–110, doi: 10.1146/annurev-ento-112408-085446.
56.
Williamson B.N., Fischer R.J., Lopez J.E., Ebihara H., Schwan T.G. Prevalence and strains of Colorado tick fever virus in Rocky Mountain wood ticks in the Bitterroot Valley, Montana. Vector Borne Zoonotic Dis. 2019; 19(9): 694–702, doi: 10.1089/vbz.2018.2407.
57.
Padgett K.A., Kjemtrup A., Novak M., Velez J.O., Panella N. Colorado tick fever virus in the Far West: forgotten, but not gone. Vector Borne Zoonotic Dis. 2022; 22(8): 443–448, doi: 10.1089/vbz.2022.0018.
58.
Pecina C.A. Tick paralysis. Semin. Neurol. 2012; 32(5): 531–532, doi: 10.1055/s-0033-1334474.
59.
Molins C.R., Ashton L.V., Wormser G.P., Andre B.G., Hess A.M., Delorey M.J. et al. Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci. Transl. Med. 2017; 9(403): eaal2717, doi: 10.1126/scitranslmed.aal2717.
60.
Seo J.W., Kim D., Yun N., Kim D.M. Clinical update of severe fever with thrombocytopenia syndrome. Viruses 2021; 13(7): 1213, doi: 10.3390/v13071213.
61.
Moniuszko A., Dunaj J., Czupryna P., Zajkowska J., Pancewicz S. Neoehrlichiosis – a new tick-borne disease – is there a threat in Poland? Przegl. Epidemiol. 2015; 69(1): 23–26, 131–133.
62.
Tyrakowska-Dadełło Z., Tarasów E., Janusek D., Moniuszko-Malinowska A., Zajkowska J., Pancewicz S. Brain perfusion alterations in tick-borne encephalitis-preliminary report. Int. J. Infect. Dis. 2018; 68: 26–30, doi: 10.1016/j.ijid.2018.01.002.
63.
Eleftheriou A., Lundin F., Petropoulos E.A. Tick-borne encephalitis: stroke-like presentation. J. Stroke Cerebrovasc. Dis. 2019; 28(8): e119–e122, doi: 10.1016/j.jstrokecerebrovasdis.2019.05.028.
64.
Tarfarosh S.F., Manzoor M. Neurological manifestations of Brucellosis in an Indian population. Cureus 2016; 8(7): e684, doi: 10.7759/cureus.684.
65.
Bukhari E.E. Pediatric brucellosis: An update review for the new millennium. Saudi Med. J. 2018; 39(4): 336–341, doi: 10.15537/smj.2018.4.21896.
66.
Choroby zakaźne i zatrucia w Polsce rok 2017 (Tabele). Narodowy Instytut Zdrowia Publicznego – Państwowy Instytut Badawczy [online] https://epibaza.pzh.gov.pl/cho... [accessed on 24 January 2024].
67.
Cai X., Ebell M.H., Haines L. Accuracy of signs, symptoms, and hematologic parameters for the diagnosis of infectious mononucleosis: A systematic review and meta-analysis. J. Am. Board Fam. Med. 2021; 34(6): 1141–1156, doi: 10.3122/jabfm.2021.06.210217.
68.
Arslan F., Karagöz E., Beköz H.S., Ceylan B., Mert A. Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis presenting with acute sensorineural hearing loss: a case report and review of the literature. Infez. Med. 2017; 25(3): 277–280.
69.
Simon L.V., Hashmi M.F., Torp K.D. Yellow fever. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan, https://www.ncbi.nlm.nih.gov/b... [accessed on 6 June 6 2022].
70.
Douam F., Ploss A. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018; 26(11): 913–928, doi: 10.1016/j.tim.2018.05.012.
71.
Yellow fever. World Health Organization, 31 May 2023 [online] https://www.who.int/news-room/... [accessed on 6 June 2022].
72.
Tattevin P., Tchamgoué S., Belem A., Bénézit F., Pronier C., Revest M. Aseptic meningitis. Rev. Neurol. (Paris) 2019; 175(7–8): 475–480, doi: 10.1016/j.neurol.2019.07.005.
73.
Wright W.F., Pinto C.N., Palisoc K., Baghli S. Viral (aseptic) meningitis: A review. J. Neurol. Sci. 2019; 398: 176–183, doi: 10.1016/j.jns.2019.01.050.
74.
Clark M.B., Schaefer T.J. West Nile virus. 2022 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan, https://www.ncbi.nlm.nih.gov/b... [accessed on 6 June 2022].
75.
Shin A., Tukhanova N., Ndenkeh J. Jr, Shapiyeva Z., Yegemberdiyeva R., Yeraliyeva L. et al. Tick-borne encephalitis virus and West-Nile fever virus as causes of serous meningitis of unknown origin in Kazakhstan. Zoonoses Public Health 2022; 69(5): 514–525, doi: 10.1111/zph.12941.
76.
Schwarz L., Akbari N., Prüss H., Meisel A., Scheibe F. Clinical characteristics, treatments, outcome, and prognostic factors of severe autoimmune encephalitis in the intensive care unit: Standard treatment and the value of additional plasma cell-depleting escalation therapies for treatment‐refractory patients. Eur. J. Neurol. 2023; 30(2): 474–489, doi: 10.1111/ene.15585.
77.
Machado S., Pinto A.N., Irani S.R. What should you know about limbic encephalitis? Arq. Neuropsiquiatr. 2012; 70(10): 817–822, doi: 10.1590/S0004-282x2012001000012.
78.
Lotric-Furlan S., Avsic-Zupanc T., Strle F. An abortive form of tick-borne encephalitis (TBE): a rare clinical manifestation of infection with TBE virus. Wien. Klin. Wochenschr. 2002; 114(13–14): 627–629.
79.
Logina I., Krumina A., Karelis G., Elsone L., Viksna L., Rozentale B. et al. Clinical features of double infection with tick-borne encephalitis and Lyme borreliosis transmitted by tick bite. J. Neurol. Neurosurg. Psychiatry 2006; 77(12): 1350–1353, doi: 10.1136/jnnp.2004.060731.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.