Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
The influence of betulin and its derivatives on the expression of TNF and its receptors in RPTEC cells
1
Department of Molecular Biology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Corresponding author
Joanna Gola
Zakład Biologii Molekularnej, Wydział Nauk Farmaceutycznych w Sosnowcu ŚUM, ul. Jedności 8, 41-200 Sosnowiec
Zakład Biologii Molekularnej, Wydział Nauk Farmaceutycznych w Sosnowcu ŚUM, ul. Jedności 8, 41-200 Sosnowiec
Ann. Acad. Med. Siles. 2025;79:237-245
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Despite their promising anticancer properties, betulin derivatives may have serious side effects, including nephrotoxicity. Tumor necrosis factor (TNF) and its receptors may play crucial roles in renal cells’ reaction to these compounds. The aim of this study was to examine the effect of the derivatives EB5 and ECH147 on renal cell expression of TNF and its receptors.
Material and methods:
Human renal proximal tubule epithelial cells (RPTECs) were treated with betulin, EB5, and ECH147, as well as cisplatin and 5-fluorouracil. The transcript levels of the genes TNF, TNFR1, and TNFR2 were assessed using real-time RT-qPCR. Protein concentrations in the culture media were determined using ELISA.
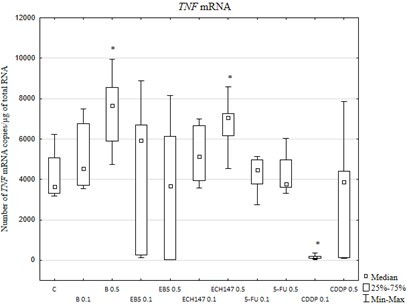
Results:
The transcriptional activity of the gene TNF was induced in cells treated with 0.5 µg/mL betulin or ECH147. Similar changes in transcriptional activity were observed for TNFR1. Betulin and its derivatives strongly inhibited the expression of TNFR2. No TNF or sTNFR2 proteins were detected in the culture media. EB5 downregulated sTNFR1 release in comparison with the other compounds.
Conclusions:
EB5 at low concentrations may be less harmful to renal cells. The lower toxicity of EB5 may be a result of the altered expression of TNF and its receptors.
Despite their promising anticancer properties, betulin derivatives may have serious side effects, including nephrotoxicity. Tumor necrosis factor (TNF) and its receptors may play crucial roles in renal cells’ reaction to these compounds. The aim of this study was to examine the effect of the derivatives EB5 and ECH147 on renal cell expression of TNF and its receptors.
Material and methods:
Human renal proximal tubule epithelial cells (RPTECs) were treated with betulin, EB5, and ECH147, as well as cisplatin and 5-fluorouracil. The transcript levels of the genes TNF, TNFR1, and TNFR2 were assessed using real-time RT-qPCR. Protein concentrations in the culture media were determined using ELISA.
Results:
The transcriptional activity of the gene TNF was induced in cells treated with 0.5 µg/mL betulin or ECH147. Similar changes in transcriptional activity were observed for TNFR1. Betulin and its derivatives strongly inhibited the expression of TNFR2. No TNF or sTNFR2 proteins were detected in the culture media. EB5 downregulated sTNFR1 release in comparison with the other compounds.
Conclusions:
EB5 at low concentrations may be less harmful to renal cells. The lower toxicity of EB5 may be a result of the altered expression of TNF and its receptors.
FUNDING
This research was funded by the Medical University of Silesia, Katowice, Poland (grant no. PCN-1-050/N/1/I).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES (37)
1.
Hordyjewska A., Ostapiuk A., Horecka A., Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019; 18: 929–951, doi: 10.1007/s11101-019-09623-1.
2.
Amiri S., Dastghaib S., Ahmadi M., Mehrbod P., Khadem F., Behrouj H. et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020; 38: 107409, doi: 10.1016/j.biotechadv.2019.06.008.
3.
Zhou Z., Zhu C., Cai Z., Zhao F., He L., Lou X. et al. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018; 15(5): 7319–7327, doi: 10.3892/ol.2018.8183.
4.
Tsepaeva O.V., Nemtarev A.V., Abdullin T.I., Grigor’eva L.R., Kuznetsova E.V., Akhmadishina R.A. et al. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J. Nat. Prod. 2017; 80(8): 2232–2239, doi: 10.1021/acs.jnatprod.7b00105.
5.
So H.M., Eom H.J., Lee D., Kim S., Kang K.S., Lee I.K. et al. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018; 41(8): 815–822, doi: 10.1007/s12272-018-1064-9.
6.
Kommera H., Kaluderović G.N., Dittrich S., Kalbitz J., Dräger B., Mueller T. et al. Carbamate derivatives of betulinic acid and betulin with selective cytotoxic activity. Bioorg. Med. Chem. Lett. 2010; 20(11): 3409–3412, doi: 10.1016/j.bmcl.2010.04.004.
7.
Csuk R., Barthel A., Sczepek R., Siewert B., Schwarz S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. (Weinheim) 2011; 344(1): 37–49, doi: 10.1002/ardp.201000232.
8.
Bi Y., Xu J., Wu X., Ye W., Yuan S., Zhang L. Synthesis and cytotoxic activity of 17-carboxylic acid modified 23-hydroxybetulinic acid ester derivatives. Bioorg. Med. Chem. Lett. 2007; 17(5): 1475–1478, doi: 10.1016/j.bmcl.2006.09.096.
9.
Csuk R., Sczepek R., Siewert B., Nitsche C. Cytotoxic betulin-derived hydroxypropargylamines trigger apoptosis. Bioorg. Med. Chem. 2013; 21(2): 425–435, doi: 10.1016/j.bmc.2012.11.016.
10.
Yamansarov E.Y., Saltykova I.V., Kovalev S.V., Petrov R.A., Shkil’ D.O., Seleznev E.I. et al. Synthesis and cytotoxicity of new alkyne derivatives of pentacyclic triterpenoids. Russ. Chem. Bull. 2019; 68: 855–861, doi: 10.1007/s11172-019-2496-1.
11.
Boryczka S., Bębenek E., Wietrzyk J., Kempińska K., Jastrzębska M., Kusz J. et al. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013; 18(4): 4526–4543, doi: 10.3390/molecules18044526.
12.
Bębenek E., Kadela-Tomanek M., Chrobak E., Wietrzyk J., Sadowska J., Boryczka S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017; 26(1): 1–8, doi: 10.1007/s00044-016-1713-9.
13.
Orchel A., Chodurek E., Jaworska-Kik M., Paduszyński P., Kaps A., Chrobak E. et al. Anticancer activity of the acetylenic derivative of betulin phosphate involves induction of necrotic-like death in breast cancer cells in vitro. Molecules 2021; 26(3): 615, doi: 10.3390/molecules26030615.
14.
Król S.K., Bębenek E., Dmoszyńska-Graniczka M., Sławińska-Brych A., Boryczka S., Stepulak A. Acetylenic synthetic betulin derivatives inhibit Akt and Erk kinases activity, trigger apoptosis and suppress proliferation of neuroblastoma and rhabdomyosarcoma cell lines. Int. J. Mol. Sci. 2021; 22(22): 12299, doi: 10.3390/ijms222212299.
15.
Chrobak E., Bębenek E., Kadela-Tomanek M., Latocha M., Jelsch C., Wenger E. et al. Betulin phosphonates; synthesis, structure, and cytotoxic activity. Molecules 2016; 21(9): 1123, doi: 10.3390/molecules21091123.
16.
Santos M.L.C., de Brito B.B., da Silva F.A.F., Botelho A.C.D.S., de Melo F.F. Nephrotoxicity in cancer treatment: An overview. World J. Clin. Oncol. 2020; 11(4): 190–204, doi: 10.5306/wjco.v11.i4.190.
17.
Kruszniewska-Rajs C., Strzałka-Mrozik B., Kimsa-Dudek M., Synowiec-Wojtarowicz A., Chrobak E., Bębenek E. et al. The influence of betulin and its derivatives EB5 and ECH147 on the antioxidant status of human renal proximal tubule epithelial cells. Int. J. Mol. Sci. 2022; 23(5): 2524, doi: 10.3390/ijms23052524.
18.
Gough P., Myles I.A. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Front. Immunol. 2020; 11: 585880, doi: 10.3389/fimmu.2020.585880.
19.
Gola J., Strzałka-Mrozik B., Kruszniewska-Rajs C., Janiszewski A., Skowronek B., Gagoś M. et al. A new form of amphotericin B – the complex with copper (II) ions – downregulates sTNFR1 shedding and changes the activity of genes involved in TNF-induced pathways: AmB-Cu2+ downregulates sTNFR1 shedding and changes the activity of genes involved in TNF-induced pathways. Pharmacol. Rep. 2017; 69(1): 22–28, doi: 10.1016/j.pharep.2016.09.008.
20.
Munawar S., Zahoor A.F., Hussain S.M., Ahmad S., Mansha A., Parveen B. et al. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2023; 10(1): e23416, doi: 10.1016/j.heliyon.2023.e23416.
21.
Hałka J., Spaleniak S., Kade G., Antosiewicz S., Sigorski D. The nephrotoxicity of drugs used in causal oncological therapies. Curr. Oncol. 2022; 29(12): 9681–9694, doi: 10.3390/curroncol29120760.
22.
García-Carro C., Draibe J., Soler M.J. Onconephrology: update in anticancer drug-related nephrotoxicity. Nephron 2023; 147(2): 65–77, doi: 10.1159/000525029.
23.
Perazella M.A. Drug-induced acute kidney injury: diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019; 25(6): 550–557, doi: 10.1097/MCC.0000000000000653.
24.
Lin T.Y., Hsu Y.H. IL-20 in acute kidney injury: role in pathogenesis and potential as a therapeutic target. Int. J. Mol. Sci. 2020; 21(3): 1009, doi: 10.3390/ijms21031009.
25.
Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014; 2014: 360438, doi: 10.1155/2014/360438.
26.
Busch C.J., Binder C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017; 1862(4): 398–406, doi: 10.1016/j.bbalip.2016.06.016.
27.
Liu S., Zhang X., Wang J. Isovitexin protects against cisplatin-induced kidney injury in mice through inhibiting inflammatory and oxidative responses. Int. Immunopharmacol. 2020; 83: 106437, doi: 10.1016/j.intimp.2020.106437.
28.
Kubica S., Szota-Czyż J., Strzałka-Mrozik B., Adamska J., Bębenek E., Chrobak E. et al. The influence of betulin derivatives EB5 and ECH147 on the expression of selected TGFβ superfamily genes, TGFβ1, GDF15 and BMP2, in renal proximal tubule epithelial cells. Curr. Issues Mol. Biol. 2023; 45(12): 9961–9975, doi: 10.3390/cimb45120622.
29.
Tang H., Zhou H., Zhang L., Tang T., Li N. Molecular mechanism of MLCK1 inducing 5-Fu resistance in colorectal cancer cells through activation of TNFR2/NF-κB pathway. Discov. Oncol. 2024; 15(1): 159, doi: 10.1007/s12672-024-01019-8.
30.
Zhang W., Ramdas L., Shen W., Song S.W., Hu L., Hamilton S.R. Apoptotic response to 5-fluorouracil treatment is mediated by reduced polyamines, non-autocrine Fas ligand and induced tumor necrosis factor receptor 2. Cancer Biol. Ther. 2003; 2(5): 572–578, doi: 10.4161/cbt.2.5.532.
31.
Ramesh G., Reeves W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 2003; 285(4): F610-F618, doi: 10.1152/ajprenal.00101.2003.
32.
Vince J.E., Pantaki D., Feltham R., Mace P.D., Cordier S.M., Schmukle A.C. et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-κB and to prevent TNF-induced apoptosis. J. Biol. Chem. 2009; 284(51): 35906–35915, doi: 10.1074/jbc.M109.072256.
33.
Zhang H., Yan D., Shi X., Liang H., Pang Y., Qin N. et al. Transmembrane TNF-α mediates "forward" and "reverse" signaling, inducing cell death or survival via the NF-κB pathway in Raji Burkitt lymphoma cells. J. Leukoc. Biol. 2008; 84(3): 789–797, doi: 10.1189/jlb.0208078.
34.
Qu Y., Zhao G., Li H. Forward and reverse signaling mediated by transmembrane tumor necrosis factor-alpha and TNF receptor 2: potential roles in an immunosuppressive tumor microenvironment. Front. Immunol. 2017; 8: 1675, doi: 10.3389/fimmu.2017.01675.
35.
Fernández-Juárez G., Villacorta Perez J., Luño Fernández J.L., Martinez--Martinez E., Cachofeiro V., Barrio Lucia V. et al. High levels of circulating TNFR1 increase the risk of all-cause mortality and progression of renal disease in type 2 diabetic nephropathy. Nephrology (Carlton) 2017; 22(5): 354–360, doi: 10.1111/nep.12781.
36.
Kamei N., Yamashita M., Nishizaki Y., Yanagisawa N., Nojiri S., Tanaka K. et al. Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Sci. Rep. 2018; 8(1): 15302, doi: 10.1038/s41598-018-33590-w.
37.
Limonte C.P., Gao X., Bebu I., Seegmiller J.C., Karger A.B., Lorenzi G.M. et al. Associations of kidney tubular biomarkers with incident macroalbuminuria and sustained low eGFR in DCCT/EDIC. Diabetes Care 2024; 47(9): 1539–1547, doi: 10.2337/dc23-2196.
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.