Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Wpływ betuliny i jej pochodnych na ekspresję TNF i jego receptorów w komórkach RPTEC
1
Department of Molecular Biology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Joanna Gola
Zakład Biologii Molekularnej, Wydział Nauk Farmaceutycznych w Sosnowcu ŚUM, ul. Jedności 8, 41-200 Sosnowiec
Zakład Biologii Molekularnej, Wydział Nauk Farmaceutycznych w Sosnowcu ŚUM, ul. Jedności 8, 41-200 Sosnowiec
Ann. Acad. Med. Siles. 2025;79:237-245
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wstęp:
Pomimo obiecujących właściwości przeciwnowotworowych pochodne betuliny mogą powodować poważne skutki uboczne, w tym nefrotoksyczność. Czynnik martwicy nowotworu (tumor necrosis factor – TNF) i jego receptory mogą odgrywać kluczową rolę w reakcji komórek nerkowych na te związki. Celem badania było określenie wpływu pochodnych EB5 i ECH147 na ekspresję TNF i jego receptorów w komórkach nerkowych.
Materiał i metody:
Ludzkie komórki nabłonkowe kanalika proksymalnego nerki (renal proximal tubule epithelial cells – RPTECs) poddano działaniu betuliny, EB5 i ECH147, a także cisplatyny i 5-fluorouracylu. Poziomy transkryptów genów TNF, TNFR1 i TNFR2 oceniono z użyciem RT-qPCR w czasie rzeczywistym. Stężenia rozpuszczalnych form białek w podłożu hodowlanym określono za pomocą testu ELISA.
Wyniki:
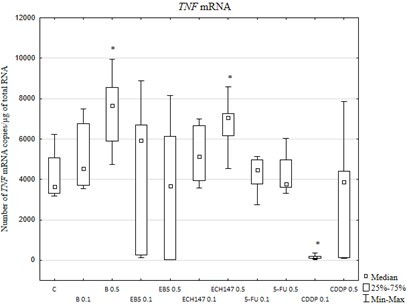
W komórkach poddanych działaniu 0,5 µg/ml betuliny lub ECH147 stwierdzono nasilenie aktywności transkrypcyjnej genu TNF. Podobne zmiany w aktywności transkrypcyjnej zaobserwowano dla genu TNFR1. Betulina i jej pochodne silnie hamowały ekspresję TNFR2. W pożywkach hodowlanych nie wykryto rozpuszczalnej formy białka TNF oraz sTNFR2. EB5 zmniejszyło uwalnianie sTNFR1 w porównaniu z innymi związkami.
Wnioski:
EB5 w niskich stężeniach może być mniej szkodliwy dla komórek nerkowych. Niższa toksyczność EB5 może wynikać ze zmienionej ekspresji TNF i jego receptorów.
Pomimo obiecujących właściwości przeciwnowotworowych pochodne betuliny mogą powodować poważne skutki uboczne, w tym nefrotoksyczność. Czynnik martwicy nowotworu (tumor necrosis factor – TNF) i jego receptory mogą odgrywać kluczową rolę w reakcji komórek nerkowych na te związki. Celem badania było określenie wpływu pochodnych EB5 i ECH147 na ekspresję TNF i jego receptorów w komórkach nerkowych.
Materiał i metody:
Ludzkie komórki nabłonkowe kanalika proksymalnego nerki (renal proximal tubule epithelial cells – RPTECs) poddano działaniu betuliny, EB5 i ECH147, a także cisplatyny i 5-fluorouracylu. Poziomy transkryptów genów TNF, TNFR1 i TNFR2 oceniono z użyciem RT-qPCR w czasie rzeczywistym. Stężenia rozpuszczalnych form białek w podłożu hodowlanym określono za pomocą testu ELISA.
Wyniki:
W komórkach poddanych działaniu 0,5 µg/ml betuliny lub ECH147 stwierdzono nasilenie aktywności transkrypcyjnej genu TNF. Podobne zmiany w aktywności transkrypcyjnej zaobserwowano dla genu TNFR1. Betulina i jej pochodne silnie hamowały ekspresję TNFR2. W pożywkach hodowlanych nie wykryto rozpuszczalnej formy białka TNF oraz sTNFR2. EB5 zmniejszyło uwalnianie sTNFR1 w porównaniu z innymi związkami.
Wnioski:
EB5 w niskich stężeniach może być mniej szkodliwy dla komórek nerkowych. Niższa toksyczność EB5 może wynikać ze zmienionej ekspresji TNF i jego receptorów.
REFERENCJE (37)
1.
Hordyjewska A., Ostapiuk A., Horecka A., Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019; 18: 929–951, doi: 10.1007/s11101-019-09623-1.
2.
Amiri S., Dastghaib S., Ahmadi M., Mehrbod P., Khadem F., Behrouj H. et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020; 38: 107409, doi: 10.1016/j.biotechadv.2019.06.008.
3.
Zhou Z., Zhu C., Cai Z., Zhao F., He L., Lou X. et al. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018; 15(5): 7319–7327, doi: 10.3892/ol.2018.8183.
4.
Tsepaeva O.V., Nemtarev A.V., Abdullin T.I., Grigor’eva L.R., Kuznetsova E.V., Akhmadishina R.A. et al. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J. Nat. Prod. 2017; 80(8): 2232–2239, doi: 10.1021/acs.jnatprod.7b00105.
5.
So H.M., Eom H.J., Lee D., Kim S., Kang K.S., Lee I.K. et al. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018; 41(8): 815–822, doi: 10.1007/s12272-018-1064-9.
6.
Kommera H., Kaluderović G.N., Dittrich S., Kalbitz J., Dräger B., Mueller T. et al. Carbamate derivatives of betulinic acid and betulin with selective cytotoxic activity. Bioorg. Med. Chem. Lett. 2010; 20(11): 3409–3412, doi: 10.1016/j.bmcl.2010.04.004.
7.
Csuk R., Barthel A., Sczepek R., Siewert B., Schwarz S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. (Weinheim) 2011; 344(1): 37–49, doi: 10.1002/ardp.201000232.
8.
Bi Y., Xu J., Wu X., Ye W., Yuan S., Zhang L. Synthesis and cytotoxic activity of 17-carboxylic acid modified 23-hydroxybetulinic acid ester derivatives. Bioorg. Med. Chem. Lett. 2007; 17(5): 1475–1478, doi: 10.1016/j.bmcl.2006.09.096.
9.
Csuk R., Sczepek R., Siewert B., Nitsche C. Cytotoxic betulin-derived hydroxypropargylamines trigger apoptosis. Bioorg. Med. Chem. 2013; 21(2): 425–435, doi: 10.1016/j.bmc.2012.11.016.
10.
Yamansarov E.Y., Saltykova I.V., Kovalev S.V., Petrov R.A., Shkil’ D.O., Seleznev E.I. et al. Synthesis and cytotoxicity of new alkyne derivatives of pentacyclic triterpenoids. Russ. Chem. Bull. 2019; 68: 855–861, doi: 10.1007/s11172-019-2496-1.
11.
Boryczka S., Bębenek E., Wietrzyk J., Kempińska K., Jastrzębska M., Kusz J. et al. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013; 18(4): 4526–4543, doi: 10.3390/molecules18044526.
12.
Bębenek E., Kadela-Tomanek M., Chrobak E., Wietrzyk J., Sadowska J., Boryczka S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017; 26(1): 1–8, doi: 10.1007/s00044-016-1713-9.
13.
Orchel A., Chodurek E., Jaworska-Kik M., Paduszyński P., Kaps A., Chrobak E. et al. Anticancer activity of the acetylenic derivative of betulin phosphate involves induction of necrotic-like death in breast cancer cells in vitro. Molecules 2021; 26(3): 615, doi: 10.3390/molecules26030615.
14.
Król S.K., Bębenek E., Dmoszyńska-Graniczka M., Sławińska-Brych A., Boryczka S., Stepulak A. Acetylenic synthetic betulin derivatives inhibit Akt and Erk kinases activity, trigger apoptosis and suppress proliferation of neuroblastoma and rhabdomyosarcoma cell lines. Int. J. Mol. Sci. 2021; 22(22): 12299, doi: 10.3390/ijms222212299.
15.
Chrobak E., Bębenek E., Kadela-Tomanek M., Latocha M., Jelsch C., Wenger E. et al. Betulin phosphonates; synthesis, structure, and cytotoxic activity. Molecules 2016; 21(9): 1123, doi: 10.3390/molecules21091123.
16.
Santos M.L.C., de Brito B.B., da Silva F.A.F., Botelho A.C.D.S., de Melo F.F. Nephrotoxicity in cancer treatment: An overview. World J. Clin. Oncol. 2020; 11(4): 190–204, doi: 10.5306/wjco.v11.i4.190.
17.
Kruszniewska-Rajs C., Strzałka-Mrozik B., Kimsa-Dudek M., Synowiec-Wojtarowicz A., Chrobak E., Bębenek E. et al. The influence of betulin and its derivatives EB5 and ECH147 on the antioxidant status of human renal proximal tubule epithelial cells. Int. J. Mol. Sci. 2022; 23(5): 2524, doi: 10.3390/ijms23052524.
18.
Gough P., Myles I.A. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Front. Immunol. 2020; 11: 585880, doi: 10.3389/fimmu.2020.585880.
19.
Gola J., Strzałka-Mrozik B., Kruszniewska-Rajs C., Janiszewski A., Skowronek B., Gagoś M. et al. A new form of amphotericin B – the complex with copper (II) ions – downregulates sTNFR1 shedding and changes the activity of genes involved in TNF-induced pathways: AmB-Cu2+ downregulates sTNFR1 shedding and changes the activity of genes involved in TNF-induced pathways. Pharmacol. Rep. 2017; 69(1): 22–28, doi: 10.1016/j.pharep.2016.09.008.
20.
Munawar S., Zahoor A.F., Hussain S.M., Ahmad S., Mansha A., Parveen B. et al. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2023; 10(1): e23416, doi: 10.1016/j.heliyon.2023.e23416.
21.
Hałka J., Spaleniak S., Kade G., Antosiewicz S., Sigorski D. The nephrotoxicity of drugs used in causal oncological therapies. Curr. Oncol. 2022; 29(12): 9681–9694, doi: 10.3390/curroncol29120760.
22.
García-Carro C., Draibe J., Soler M.J. Onconephrology: update in anticancer drug-related nephrotoxicity. Nephron 2023; 147(2): 65–77, doi: 10.1159/000525029.
23.
Perazella M.A. Drug-induced acute kidney injury: diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019; 25(6): 550–557, doi: 10.1097/MCC.0000000000000653.
24.
Lin T.Y., Hsu Y.H. IL-20 in acute kidney injury: role in pathogenesis and potential as a therapeutic target. Int. J. Mol. Sci. 2020; 21(3): 1009, doi: 10.3390/ijms21031009.
25.
Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014; 2014: 360438, doi: 10.1155/2014/360438.
26.
Busch C.J., Binder C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017; 1862(4): 398–406, doi: 10.1016/j.bbalip.2016.06.016.
27.
Liu S., Zhang X., Wang J. Isovitexin protects against cisplatin-induced kidney injury in mice through inhibiting inflammatory and oxidative responses. Int. Immunopharmacol. 2020; 83: 106437, doi: 10.1016/j.intimp.2020.106437.
28.
Kubica S., Szota-Czyż J., Strzałka-Mrozik B., Adamska J., Bębenek E., Chrobak E. et al. The influence of betulin derivatives EB5 and ECH147 on the expression of selected TGFβ superfamily genes, TGFβ1, GDF15 and BMP2, in renal proximal tubule epithelial cells. Curr. Issues Mol. Biol. 2023; 45(12): 9961–9975, doi: 10.3390/cimb45120622.
29.
Tang H., Zhou H., Zhang L., Tang T., Li N. Molecular mechanism of MLCK1 inducing 5-Fu resistance in colorectal cancer cells through activation of TNFR2/NF-κB pathway. Discov. Oncol. 2024; 15(1): 159, doi: 10.1007/s12672-024-01019-8.
30.
Zhang W., Ramdas L., Shen W., Song S.W., Hu L., Hamilton S.R. Apoptotic response to 5-fluorouracil treatment is mediated by reduced polyamines, non-autocrine Fas ligand and induced tumor necrosis factor receptor 2. Cancer Biol. Ther. 2003; 2(5): 572–578, doi: 10.4161/cbt.2.5.532.
31.
Ramesh G., Reeves W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 2003; 285(4): F610-F618, doi: 10.1152/ajprenal.00101.2003.
32.
Vince J.E., Pantaki D., Feltham R., Mace P.D., Cordier S.M., Schmukle A.C. et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-κB and to prevent TNF-induced apoptosis. J. Biol. Chem. 2009; 284(51): 35906–35915, doi: 10.1074/jbc.M109.072256.
33.
Zhang H., Yan D., Shi X., Liang H., Pang Y., Qin N. et al. Transmembrane TNF-α mediates "forward" and "reverse" signaling, inducing cell death or survival via the NF-κB pathway in Raji Burkitt lymphoma cells. J. Leukoc. Biol. 2008; 84(3): 789–797, doi: 10.1189/jlb.0208078.
34.
Qu Y., Zhao G., Li H. Forward and reverse signaling mediated by transmembrane tumor necrosis factor-alpha and TNF receptor 2: potential roles in an immunosuppressive tumor microenvironment. Front. Immunol. 2017; 8: 1675, doi: 10.3389/fimmu.2017.01675.
35.
Fernández-Juárez G., Villacorta Perez J., Luño Fernández J.L., Martinez--Martinez E., Cachofeiro V., Barrio Lucia V. et al. High levels of circulating TNFR1 increase the risk of all-cause mortality and progression of renal disease in type 2 diabetic nephropathy. Nephrology (Carlton) 2017; 22(5): 354–360, doi: 10.1111/nep.12781.
36.
Kamei N., Yamashita M., Nishizaki Y., Yanagisawa N., Nojiri S., Tanaka K. et al. Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Sci. Rep. 2018; 8(1): 15302, doi: 10.1038/s41598-018-33590-w.
37.
Limonte C.P., Gao X., Bebu I., Seegmiller J.C., Karger A.B., Lorenzi G.M. et al. Associations of kidney tubular biomarkers with incident macroalbuminuria and sustained low eGFR in DCCT/EDIC. Diabetes Care 2024; 47(9): 1539–1547, doi: 10.2337/dc23-2196.
Udostępnij
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.