Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Traceability for strengthening supply chain systems and enhancing real-time visibility:
Focus of NAFDAC on advancing vaccine traceability in Nigeria
1
National Agency for Food and Drug Administration and Control, Lagos, Nigeria
These authors had equal contribution to this work
Corresponding author
Ann. Acad. Med. Siles. 2024;78:276-281
KEYWORDS
TOPICS
ABSTRACT
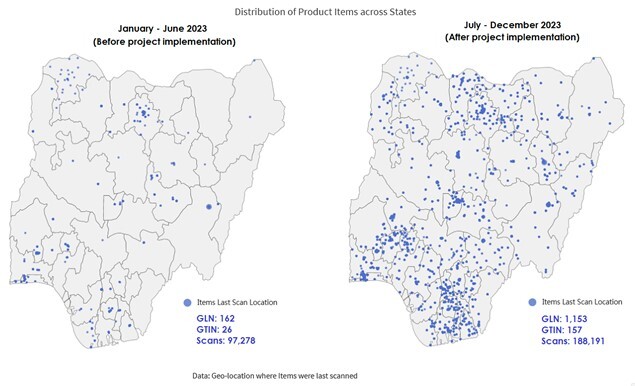
This study explores the successful implementation of activities aimed at scaling traceability for COVID-19 and routine immunization (RI) vaccines to the local government area and selected healthcare facilities in Nigeria, funded by the Bill and Melinda Gates Foundation. The study was executed by the National Agency for Food and Drug Administration and Control (NAFDAC) and sought to enhance supply chain systems by deploying advanced traceability mechanisms and ensuring real-time stock visibility. The article discusses the accomplishments, challenges, and regulatory framework of NAFDAC, emphasizing GS1 technology-driven traceability, and presents the results of the field scanning activities conducted in July 2023. The approach involved a phased public sector pilot, showcasing the feasibility and challenges of tracking vaccine movement through the supply chain. The result shows the detection of 43 unique products across 1022 facilities from a total of 110,113 scans, offering valuable insights into vaccine distributions. The strategic goals of the project aligned with developing safety surveillance systems in low- and middle-income countries (LMICs) to facilitate patient access to global health products. Similarly, significant improvement in traceability through automated data capture (barcode scanning) and expanded coverage for COVID-19 and selected RI vaccines in Nigeria was found. Against this background, the information derived from this report will build confidence in patients regarding vaccine authenticity, establish a transparent and robust supply chain, and foster pharmacovigilance capability through integration with the track-and-trace systems. Thus, the study provides invaluable insights and opportunities for global health practitioners, policymakers, and researchers to incorporate track-and-trace into regulatory systems by other national regulatory authorities.
ACKNOWLEDGEMENTS
We are grateful to the Global Steering Committee and Mr. Tom Woods, the Coordinator who fostered the Traceability Project through the second African GS1 Health Conference in Nigeria. We extend our deep appreciation to the Bill and Melinda Gates Foundation for its invaluable support in funding the project, enabling the procurement of scanners, and facilitating the training and supervision of officers across various states for the scanning activities. Our sincere gratitude also goes to the dedicated NAFDAC staff who tirelessly contributed to the success of this project, ensuring effective scanning across facilities in their respective states in collaboration with the State Cold Chain Officers, whose efforts we duly recognize. Special acknowledgment is given to the NAFDAC Traceability Office and GS1 Nigeria for their significant contribution and technical support during the implementation of this project. We thank the Director General of NAFDAC for the final edits and approval of this study.
FUNDING
Financial support from the Bill and Melinda Gates Foundation was received for this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES (5)
1.
Traceability Solutions. Keyence [online] https://www.keyence.com/ss/pro... [access on 5 February 2024].
2.
Pharmaceutical Traceability. NAFDAC [online] https://www.nafdac.gov.ng/TRAC... [access on 5 February 2024].
3.
Policy paper on traceability of medical products. World Health Organization, 18 March 2021 [online] https://www.who.int/publicatio... [access on 5 February 2024].
4.
Adeyeye M.C., Kayode J.O., Adeniran A.A., Osho F., Udokwelu W. Enabling pharmaceutical traceability in the Nigerian supply chain using GS1 global standards: lean traceability including in-country serialization of COVID-19 vaccines. J. Regul. Sci. 2023; 11(1): 1–14, doi: 10.21423/JRS.REGSCI.111252.
5.
Lacey S., Mitchell A.D. Regulatory cooperation for vaccines: the Asia-Pacific and beyond. Asian Int. Stud. Rev. 2023; 24(1): 74–102, doi: 10.1163/2667078x-bja10025.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.