Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Traceability for strengthening supply chain systems and enhancing real-time visibility:
Focus of NAFDAC on advancing vaccine traceability in Nigeria
1
National Agency for Food and Drug Administration and Control, Lagos, Nigeria
Zaznaczeni autorzy mieli równy wkład w przygotowanie tego artykułu
Autor do korespondencji
Ann. Acad. Med. Siles. 2024;78:276-281
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
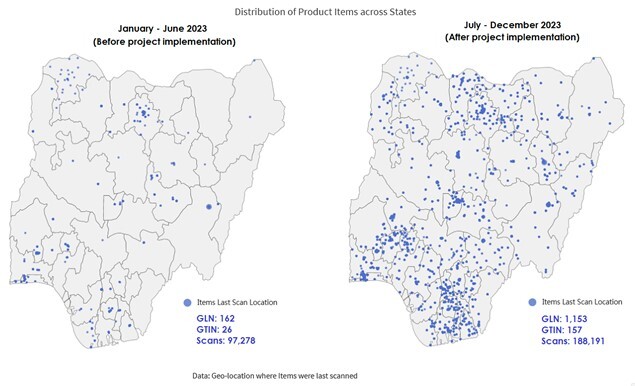
This study explores the successful implementation of activities aimed at scaling traceability for COVID-19 and routine immunization (RI) vaccines to the local government area and selected healthcare facilities in Nigeria, funded by the Bill and Melinda Gates Foundation. The study was executed by the National Agency for Food and Drug Administration and Control (NAFDAC) and sought to enhance supply chain systems by deploying advanced traceability mechanisms and ensuring real-time stock visibility. The article discusses the accomplishments, challenges, and regulatory framework of NAFDAC, emphasizing GS1 technology-driven traceability, and presents the results of the field scanning activities conducted in July 2023. The approach involved a phased public sector pilot, showcasing the feasibility and challenges of tracking vaccine movement through the supply chain. The result shows the detection of 43 unique products across 1022 facilities from a total of 110,113 scans, offering valuable insights into vaccine distributions. The strategic goals of the project aligned with developing safety surveillance systems in low- and middle-income countries (LMICs) to facilitate patient access to global health products. Similarly, significant improvement in traceability through automated data capture (barcode scanning) and expanded coverage for COVID-19 and selected RI vaccines in Nigeria was found. Against this background, the information derived from this report will build confidence in patients regarding vaccine authenticity, establish a transparent and robust supply chain, and foster pharmacovigilance capability through integration with the track-and-trace systems. Thus, the study provides invaluable insights and opportunities for global health practitioners, policymakers, and researchers to incorporate track-and-trace into regulatory systems by other national regulatory authorities.
REFERENCJE (5)
1.
Traceability Solutions. Keyence [online] https://www.keyence.com/ss/pro... [access on 5 February 2024].
2.
Pharmaceutical Traceability. NAFDAC [online] https://www.nafdac.gov.ng/TRAC... [access on 5 February 2024].
3.
Policy paper on traceability of medical products. World Health Organization, 18 March 2021 [online] https://www.who.int/publicatio... [access on 5 February 2024].
4.
Adeyeye M.C., Kayode J.O., Adeniran A.A., Osho F., Udokwelu W. Enabling pharmaceutical traceability in the Nigerian supply chain using GS1 global standards: lean traceability including in-country serialization of COVID-19 vaccines. J. Regul. Sci. 2023; 11(1): 1–14, doi: 10.21423/JRS.REGSCI.111252.
5.
Lacey S., Mitchell A.D. Regulatory cooperation for vaccines: the Asia-Pacific and beyond. Asian Int. Stud. Rev. 2023; 24(1): 74–102, doi: 10.1163/2667078x-bja10025.
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.