Current issue
About the Journal
Scientific Council
Editorial Board
Regulatory and archival policy
Code of publishing ethics
Publisher
Information about the processing of personal data in relation to cookies and newsletter subscription
Archive
For Authors
For Reviewers
Contact
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
Links
Sklep Wydawnictwa SUM
Biblioteka Główna SUM
Śląski Uniwersytet Medyczny w Katowicach
Privacy policy
Accessibility statement
Reviewers
Annals reviewers in 2025
Annals reviewers in 2024
Annals reviewers in 2023
Annals reviewers in 2022
Annals reviewers in 2021
Annals reviewers in 2020
Annals reviewers in 2019
Annals reviewers in 2018
Annals reviewers in 2017
Annals reviewers in 2016
Annals reviewers in 2015
Annals reviewers in 2014
Annals reviewers in 2013
Annals reviewers in 2012
The significance and prognostic, diagnostic, and therapeutic potential of selected paracrine factors in type 2 diabetes
1
Students’ Scientific Club, Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
The John Paul II Pediatric Center, Sosnowiec, Poland
Corresponding author
Klaudia Stocerz
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:179-186
KEYWORDS
TOPICS
ABSTRACT
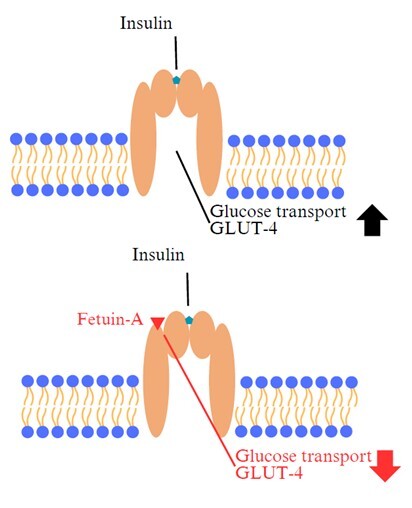
Type 2 diabetes mellitus (T2DM) is one of the most common modern “disease of affluence”, characterized by hyperglycemia resulting from defective insulin secretion by pancreatic β cells and/or impaired tissue sensitivity to insulin. In Poland, the incidence of this metabolic disease is 5–8% and is still increasing. The consequences of the development of T2DM include, among others: changes in cardiovascular system, retinopathy, nephropathy and neuropathy. Due to medical progress, it is easier to control glycemia, as well as to predict the occurrence of this disease or the dynamics of its development and consequences. The molecules discussed in the article: growth differentiation factor 15 (GDF-15), mesencephalic astrocyte-derived neurotrophic factor (MANF) and fetuin-A, represent great promise both in the diagnosis and treatment of not only T2DM, but also obesity, which often accompanies T2DM. Most of the studies reported in the literature were performed on animal models, mainly mouse. This article presents arguments supporting the potential usefulness of the above-mentioned paracrine factors for prognostic, diagnostic and therapeutic purposes.
REFERENCES (47)
1.
Harreiter J., Roden M. Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023). [Article in German]. Wien. Klin. Wochenschr. 2023; 135(Suppl 1): 7–17, doi: 10.1007/s00508-022-02122-y.
2.
Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020; 21(17): 6275, doi: 10.3390/ijms21176275.
3.
Pippitt K., Li M., Gurgle H.E. Diabetes mellitus: screening and diagnosis. Am. Fam. Physician 2016; 93(2): 103–109. Erratum in: Am. Fam. Physician 2016; 94(7): 533.
4.
Piechota W., Krzesiński P. Czynnik różnicowania wzrostu 15 (GDF-15) w ocenie ryzyka sercowo-naczyniowego. Pediatr. Med. Rodz. 2018; 14(1): 9–19, doi: 10.15557/PiMR.2018.0001.
5.
Kempf T., Eden M., Strelau J., Naguib M., Willenbockel C., Tongers J. et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006; 98(3): 351–360, doi: 10.1161/01.RES.0000202805.73038.48.
6.
Verhamme F.M., Freeman C.M., Brusselle G.G., Bracke K.R., Curtis J.L. GDF-15 in pulmonary and critical care medicine. Am. J. Respir. Cell Mol. Biol. 2019; 60(6): 621–628, doi: 10.1165/rcmb.2018-0379TR.
7.
Niu Y., Zhang W., Shi J., Liu Y., Zhang H., Lin N. et al. The relationship between circulating growth differentiation factor 15 levels and diabetic retinopathy in patients with type 2 diabetes. Front. Endocrinol. (Lausanne) 2021; 12: 627395, doi: 10.3389/fendo.2021.627395.
8.
Hellemons M.E., Mazagova M., Gansevoort R.T., Henning R.H., de Zeeuw D., Bakker S.J.L. et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care 2012; 35(11): 2340–2346, doi: 10.2337/dc12-0180.
9.
Lajer M., Jorsal A., Tarnow L., Parving H.H., Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care 2010; 33(7): 1567–1572, doi: 10.2337/dc09-2174.
10.
He X., Su J., Ma X., Lu W., Zhu W., Wang Y. et al. The association between serum growth differentiation factor 15 levels and lower extremity atherosclerotic disease is independent of body mass index in type 2 diabetes. Cardiovasc. Diabetol. 2020; 19(1): 40, doi: 10.1186/s12933-020-01020-9.
11.
Chung J.O., Park S.Y., Cho D.H., Chung D.J., Chung M.Y. Relationship between plasma growth differentiation factor-15 levels diabetic retinopathy in individuals with type 2 diabetes. Sci. Rep. 2020; 10(1): 20568, doi: 10.1038/s41598-020-77584-z.
12.
Ilhan H.D., Bilgin A.B., Toylu A., Dogan M.E., Apaydin K.C. The expression of GDF-15 in the human vitreous in the presence of retinal pathologies with an inflammatory component. Ocul. Immunol. Inflamm. 2016; 24(2): 178–183, doi: 10.3109/09273948.2014.981549.
13.
Charalambous P., Wang X., Thanos S., Schober A., Unsicker K. Regulation and effects of GDF-15 in the retina following optic nerve crush. Cell Tissue Res. 2013; 353(1): 1–8, doi: 10.1007/s00441-013-1634-6.
14.
Coll A.P., Chen M., Taskar P., Rimmington D., Patel S., Tadross J.A. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020; 578(7795): 444–448, doi: 10.1038/s41586-019-1911-y.
15.
Cai L., Li C., Wang Y., Mo Y., Yin J., Ma X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab. Syndr. Obes. 2021; 14: 1195–1202, doi: 10.2147/DMSO.S302033.
16.
Gao F., Li C., Wang Y., Lu J., Lu W., Zhou J. et al. Growth differentiation factor 15 is not associated with glycemic control in patients with type 2 diabetes mellitus treated with metformin: a post-hoc analysis of AIM study. BMC Endocr. Disord. 2022; 22(1): 256, doi: 10.1186/s12902-022-01176-3.
17.
Govaere O., Cockell S., Tiniakos D., Queen R., Younes R., Vacca M. et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020; 12(572): eaba4448, doi: 10.1126/scitranslmed.aba4448.
18.
Petrova P., Raibekas A., Pevsner J., Vigo N., Anafi M., Moore M.K. et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003; 20(2): 173–188, doi: 10.1385/JMN:20:2:173.
19.
Yang S., Li S., Li X.J. MANF: a new player in the control of energy homeostasis, and beyond. Front. Physiol. 2018; 9: 1725, doi: 10.3389/fphys.2018.01725.
20.
Yu Y., Liu D.Y., Chen X.S., Zhu L., Wan L.H. MANF: a novel endoplasmic reticulum stress response protein–the role in neurological and metabolic disorders. Oxid. Med. Cell. Longev. 2021; 2021: 6467679, doi: 10.1155/2021/6467679.
21.
Hellman M., Arumäe U., Yu L.Y., Lindholm P., Peränen J., Saarma M. et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J. Biol. Chem. 2011; 286(4): 2675–2680, doi: 10.1074/jbc.M110.146738.
22.
Tang Q., Li Y., He J. MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol. Metab. 2022; 33(4): 236–246, doi: 10.1016/j.tem.2022.01.001.
23.
Henderson M.J., Richie C.T., Airavaara M., Wang Y., Harvey B.K. Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J. Biol. Chem. 2013; 288(6): 4209–4225, doi: 10.1074/jbc.M112.400648.
24.
Yagi T., Asada R., Kanekura K., Eesmaa A., Lindahl M., Saarma M. et al. Neuroplastin modulates anti-inflammatory effects of MANF. iScience 2020; 23(12): 101810, doi: 10.1016/j.isci.2020.101810.
25.
Ikegami H., Babaya N., Noso S. β-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021; 12(9): 1526–1539, doi: 10.1111/jdi.13576.
26.
Cunha D.A., Cito M., Grieco F.A., Cosentino C., Danilova T., Ladrière L. et al. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J. Biol. Chem. 2017; 292(36): 14977–14988, doi: 10.1074/jbc.M116.769877.
27.
Hakonen E., Chandra V., Fogarty C.L., Yu N.Y., Ustinov J., Katayama S. et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia 2018; 61(10): 2202–2214, doi: 10.1007/s00125-018-4687-y.
28.
Danilova T., Galli E., Pakarinen E., Palm E., Lindholm P., Saarma M. et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front. Endocrinol. (Lausanne) 2019; 10: 765, doi: 10.3389/fendo.2019.00765.
29.
Wu T., Liu Q., Li Y., Li H., Chen L., Yang X. et al. Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J. Exp. Med. 2021; 218(6): e20201203, doi: 10.1084/jem.20201203.
30.
Yang S., Yang H., Chang R., Yin P., Yang Y., Yang W. et al. MANF regulates hypothalamic control of food intake and body weight. Nat. Commun. 2017; 8(1): 579, doi: 10.1038/s41467-017-00750-x.
31.
Sousa-Victor P., Neves J., Cedron-Craft W., Ventura P.B., Liao C.Y., Riley R.R. et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat. Metab. 2019; 1(2): 276–290, doi: 10.1038/s42255-018-0023-6.
32.
Pakarinen E., Lindholm P., Saarma M., Lindahl M. CDNF and MANF regulate ER stress in a tissue-specific manner. Cell. Mol. Life Sci. 2022; 79(2): 124, doi: 10.1007/s00018-022-04157-w.
33.
Lindahl M., Danilova T., Palm E., Lindholm P., Võikar V., Hakonen E. et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell. Rep. 2014; 7(2): 366–375, doi: 10.1016/j.celrep.2014.03.023.
34.
Wang C., Peng J.J., Miao H., Liu D.F., Zhang L.L. Decreased plasma MANF levels are associated with type 2 diabetes. Biomed. Environ. Sci. 2021; 34(3): 236–40, doi: 10.3967/bes2021.030.
35.
Wu T., Zhang F., Yang Q., Zhang Y., Liu Q., Jiang W. et al. Circulating mesencephalic astrocyte-derived neurotrophic factor is increased in newly diagnosed prediabetic and diabetic patients, and is associated with insulin resistance. Endocr. J. 2017; 64(4): 403–410, doi: 10.1507/endocrj.EJ16-0472.
36.
Ochieng J., Nangami G., Sakwe A., Moye C., Alvarez J., Whalen D. et al. Impact of fetuin-A (AHSG) on tumor progression and type 2 diabetes. Int. J. Mol. Sci. 2018; 19(8): 2211, doi: 10.3390/ijms19082211.
37.
Wang Y., Koh W.P., Jensen M.K., Yuan J.M., Pan A. Plasma fetuin-A levels and risk of type 2 diabetes mellitus in a Chinese population: a nested case-control study. Diabetes Metab. J. 2019; 43(4): 474–486, doi: 10.4093/dmj.2018.0171.
38.
Ricken F., Can A.D., Gräber S., Häusler M., Jahnen-Dechent W. Post-translational modifications glycosylation and phosphorylation of the major hepatic plasma protein fetuin-A are associated with CNS inflammation in children. PLoS One 2022; 17(10): e0268592, doi: 10.1371/journal.pone.0268592.
39.
Harbuwono D.S., Sazli B.I., Kurniawan F., Darmowidjojo B., Koesnoe S., Tahapary D.L. The impact of Ramadan fasting on fetuin-A level in type 2 diabetes mellitus. Heliyon 2021; 7(5): e06773, doi: 10.1016/j.heliyon.2021.e06773.
40.
Roshanzamir F., Miraghajani M., Rouhani M.H., Mansourian M., Ghiasvand R., Safavi S.M. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J. Endocrinol. Invest. 2018; 41(1): 33–47, doi: 10.1007/s40618-017-0697-8.
41.
Guo V.Y., Cao B., Cai C., Cheng K.K., Cheung B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2018; 55(1): 87–98, doi: 10.1007/s00592-017-1068-9.
42.
Robinson K.N., Teran-Garcia M. From infancy to aging: Biological and behavioral modifiers of fetuin-A. Biochimie 2016; 124: 141–149, doi: 10.1016/j.biochi.2015.12.016.
43.
Chekol Abebe E., Tilahun Muche Z., Behaile T/Mariam A., Mengie Ayele T., Mekonnen Agidew M., Teshome Azezew M. et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front. Cell Dev. Biol. 2022; 10: 945287, doi: 10.3389/fcell.2022.945287.
44.
Selvaraju V., Babu J.R., Geetha T. Multiplexed measurements of salivary fetuin-A, insulin, and adiponectin as potential non-invasive biomarkers in childhood obesity. Cytokine 2022; 153: 155843, doi: 10.1016/j.cyto.2022.155843.
45.
Sujana C., Huth C., Zierer A., Meesters S., Sudduth-Klinger J., Koenig W. et al. Association of fetuin-A with incident type 2 diabetes: results from the MONICA/KORA Augsburg study and a systematic meta-analysis. Eur. J. Endocrinol. 2018; 178(4): 389–398, doi: 10.1530/EJE-17-1053.
46.
Kim J.E., Kim J.S., Jo M.J., Cho E., Ahn S.Y., Kwon Y.J. et al. The roles and associated mechanisms of adipokines in development of metabolic syndrome. Molecules 2022; 27(2): 334, doi: 10.3390/molecules27020334.
47.
Bourebaba L., Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J. Clin. Med. 2019; 8(12): 2033, doi: 10.3390/jcm8122033.
Share
RELATED ARTICLE
The Medical University of Silesia in Katowice, as the Operator of the annales.sum.edu.pl website, processes personal data collected when visiting the website. The function of obtaining information about Users and their behavior is carried out by voluntarily entered information in forms, saving cookies in end devices, as well as by collecting web server logs, which are in the possession of the website Operator. Data, including cookies, are used to provide services in accordance with the Privacy policy.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.
You can consent to the processing of data for these purposes, refuse consent or access more detailed information.