Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Znaczenie oraz potencjał prognostyczny, diagnostyczny i terapeutyczny wybranych czynników parakrynnych w cukrzycy typu 2
1
Students’ Scientific Club, Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
The John Paul II Pediatric Center, Sosnowiec, Poland
Autor do korespondencji
Klaudia Stocerz
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:179-186
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
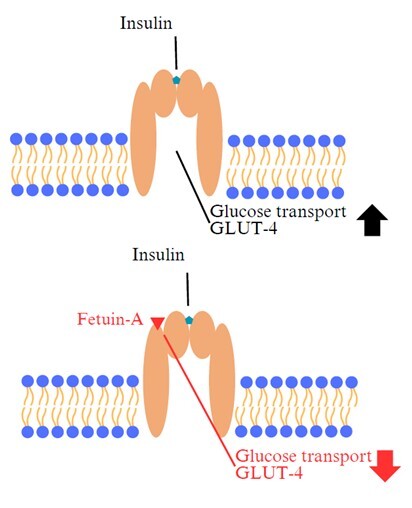
Cukrzyca typu 2 (type 2 diabetes mellitus – T2DM) to jedna z najczęściej występujących współczesnych chorób cywilizacyjnych, odznaczająca się hiperglikemią będącą skutkiem wadliwego wydzielania insuliny przez komórki β trzustki i/lub niewłaściwą wrażliwością tkanek na insulinę. W Polsce zapadalność na tę chorobę metaboliczną wynosi 5–8% i wciąż ma tendencję wzrostową. Konsekwencją rozwoju T2DM są m.in. zmiany w układzie sercowo-naczyniowym, retinopatia, nefropatia i neuropatia. Dzięki postępowi medycyny ułatwione jest kontrolowanie glikemii, jak również predykcja wystąpienia choroby bądź dynamiki jej rozwoju i następstw. Z omówionymi w artykule molekułami – różnicującym czynnikiem wzrostu 15 (growth differentiation factor 15 – GDF-15), śródmózgowym czynnikiem neurotroficznym pochodzenia astrocytarnego (mesencephalic astrocyte-derived neurotrophic factor – MANF) i fetuiną-A – wiążą się duże nadzieje zarówno w diagnostyce, jak i leczeniu nie tylko T2DM, ale również otyłości, która niejednokrotnie towarzyszy T2DM. Większość badań opisywanych w literaturze wykonywana była na modelu zwierzęcym, głównie mysim. Niniejszy artykuł przedstawia argumenty przemawiające za potencjalną użytecznością wspomnianych czynników parakrynnych w celach prognostycznych, diagnostycznych, jak również leczniczych.
REFERENCJE (47)
1.
Harreiter J., Roden M. Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023). [Article in German]. Wien. Klin. Wochenschr. 2023; 135(Suppl 1): 7–17, doi: 10.1007/s00508-022-02122-y.
2.
Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020; 21(17): 6275, doi: 10.3390/ijms21176275.
3.
Pippitt K., Li M., Gurgle H.E. Diabetes mellitus: screening and diagnosis. Am. Fam. Physician 2016; 93(2): 103–109. Erratum in: Am. Fam. Physician 2016; 94(7): 533.
4.
Piechota W., Krzesiński P. Czynnik różnicowania wzrostu 15 (GDF-15) w ocenie ryzyka sercowo-naczyniowego. Pediatr. Med. Rodz. 2018; 14(1): 9–19, doi: 10.15557/PiMR.2018.0001.
5.
Kempf T., Eden M., Strelau J., Naguib M., Willenbockel C., Tongers J. et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006; 98(3): 351–360, doi: 10.1161/01.RES.0000202805.73038.48.
6.
Verhamme F.M., Freeman C.M., Brusselle G.G., Bracke K.R., Curtis J.L. GDF-15 in pulmonary and critical care medicine. Am. J. Respir. Cell Mol. Biol. 2019; 60(6): 621–628, doi: 10.1165/rcmb.2018-0379TR.
7.
Niu Y., Zhang W., Shi J., Liu Y., Zhang H., Lin N. et al. The relationship between circulating growth differentiation factor 15 levels and diabetic retinopathy in patients with type 2 diabetes. Front. Endocrinol. (Lausanne) 2021; 12: 627395, doi: 10.3389/fendo.2021.627395.
8.
Hellemons M.E., Mazagova M., Gansevoort R.T., Henning R.H., de Zeeuw D., Bakker S.J.L. et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care 2012; 35(11): 2340–2346, doi: 10.2337/dc12-0180.
9.
Lajer M., Jorsal A., Tarnow L., Parving H.H., Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care 2010; 33(7): 1567–1572, doi: 10.2337/dc09-2174.
10.
He X., Su J., Ma X., Lu W., Zhu W., Wang Y. et al. The association between serum growth differentiation factor 15 levels and lower extremity atherosclerotic disease is independent of body mass index in type 2 diabetes. Cardiovasc. Diabetol. 2020; 19(1): 40, doi: 10.1186/s12933-020-01020-9.
11.
Chung J.O., Park S.Y., Cho D.H., Chung D.J., Chung M.Y. Relationship between plasma growth differentiation factor-15 levels diabetic retinopathy in individuals with type 2 diabetes. Sci. Rep. 2020; 10(1): 20568, doi: 10.1038/s41598-020-77584-z.
12.
Ilhan H.D., Bilgin A.B., Toylu A., Dogan M.E., Apaydin K.C. The expression of GDF-15 in the human vitreous in the presence of retinal pathologies with an inflammatory component. Ocul. Immunol. Inflamm. 2016; 24(2): 178–183, doi: 10.3109/09273948.2014.981549.
13.
Charalambous P., Wang X., Thanos S., Schober A., Unsicker K. Regulation and effects of GDF-15 in the retina following optic nerve crush. Cell Tissue Res. 2013; 353(1): 1–8, doi: 10.1007/s00441-013-1634-6.
14.
Coll A.P., Chen M., Taskar P., Rimmington D., Patel S., Tadross J.A. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020; 578(7795): 444–448, doi: 10.1038/s41586-019-1911-y.
15.
Cai L., Li C., Wang Y., Mo Y., Yin J., Ma X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab. Syndr. Obes. 2021; 14: 1195–1202, doi: 10.2147/DMSO.S302033.
16.
Gao F., Li C., Wang Y., Lu J., Lu W., Zhou J. et al. Growth differentiation factor 15 is not associated with glycemic control in patients with type 2 diabetes mellitus treated with metformin: a post-hoc analysis of AIM study. BMC Endocr. Disord. 2022; 22(1): 256, doi: 10.1186/s12902-022-01176-3.
17.
Govaere O., Cockell S., Tiniakos D., Queen R., Younes R., Vacca M. et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020; 12(572): eaba4448, doi: 10.1126/scitranslmed.aba4448.
18.
Petrova P., Raibekas A., Pevsner J., Vigo N., Anafi M., Moore M.K. et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003; 20(2): 173–188, doi: 10.1385/JMN:20:2:173.
19.
Yang S., Li S., Li X.J. MANF: a new player in the control of energy homeostasis, and beyond. Front. Physiol. 2018; 9: 1725, doi: 10.3389/fphys.2018.01725.
20.
Yu Y., Liu D.Y., Chen X.S., Zhu L., Wan L.H. MANF: a novel endoplasmic reticulum stress response protein–the role in neurological and metabolic disorders. Oxid. Med. Cell. Longev. 2021; 2021: 6467679, doi: 10.1155/2021/6467679.
21.
Hellman M., Arumäe U., Yu L.Y., Lindholm P., Peränen J., Saarma M. et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J. Biol. Chem. 2011; 286(4): 2675–2680, doi: 10.1074/jbc.M110.146738.
22.
Tang Q., Li Y., He J. MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol. Metab. 2022; 33(4): 236–246, doi: 10.1016/j.tem.2022.01.001.
23.
Henderson M.J., Richie C.T., Airavaara M., Wang Y., Harvey B.K. Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J. Biol. Chem. 2013; 288(6): 4209–4225, doi: 10.1074/jbc.M112.400648.
24.
Yagi T., Asada R., Kanekura K., Eesmaa A., Lindahl M., Saarma M. et al. Neuroplastin modulates anti-inflammatory effects of MANF. iScience 2020; 23(12): 101810, doi: 10.1016/j.isci.2020.101810.
25.
Ikegami H., Babaya N., Noso S. β-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021; 12(9): 1526–1539, doi: 10.1111/jdi.13576.
26.
Cunha D.A., Cito M., Grieco F.A., Cosentino C., Danilova T., Ladrière L. et al. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J. Biol. Chem. 2017; 292(36): 14977–14988, doi: 10.1074/jbc.M116.769877.
27.
Hakonen E., Chandra V., Fogarty C.L., Yu N.Y., Ustinov J., Katayama S. et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia 2018; 61(10): 2202–2214, doi: 10.1007/s00125-018-4687-y.
28.
Danilova T., Galli E., Pakarinen E., Palm E., Lindholm P., Saarma M. et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front. Endocrinol. (Lausanne) 2019; 10: 765, doi: 10.3389/fendo.2019.00765.
29.
Wu T., Liu Q., Li Y., Li H., Chen L., Yang X. et al. Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J. Exp. Med. 2021; 218(6): e20201203, doi: 10.1084/jem.20201203.
30.
Yang S., Yang H., Chang R., Yin P., Yang Y., Yang W. et al. MANF regulates hypothalamic control of food intake and body weight. Nat. Commun. 2017; 8(1): 579, doi: 10.1038/s41467-017-00750-x.
31.
Sousa-Victor P., Neves J., Cedron-Craft W., Ventura P.B., Liao C.Y., Riley R.R. et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat. Metab. 2019; 1(2): 276–290, doi: 10.1038/s42255-018-0023-6.
32.
Pakarinen E., Lindholm P., Saarma M., Lindahl M. CDNF and MANF regulate ER stress in a tissue-specific manner. Cell. Mol. Life Sci. 2022; 79(2): 124, doi: 10.1007/s00018-022-04157-w.
33.
Lindahl M., Danilova T., Palm E., Lindholm P., Võikar V., Hakonen E. et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell. Rep. 2014; 7(2): 366–375, doi: 10.1016/j.celrep.2014.03.023.
34.
Wang C., Peng J.J., Miao H., Liu D.F., Zhang L.L. Decreased plasma MANF levels are associated with type 2 diabetes. Biomed. Environ. Sci. 2021; 34(3): 236–40, doi: 10.3967/bes2021.030.
35.
Wu T., Zhang F., Yang Q., Zhang Y., Liu Q., Jiang W. et al. Circulating mesencephalic astrocyte-derived neurotrophic factor is increased in newly diagnosed prediabetic and diabetic patients, and is associated with insulin resistance. Endocr. J. 2017; 64(4): 403–410, doi: 10.1507/endocrj.EJ16-0472.
36.
Ochieng J., Nangami G., Sakwe A., Moye C., Alvarez J., Whalen D. et al. Impact of fetuin-A (AHSG) on tumor progression and type 2 diabetes. Int. J. Mol. Sci. 2018; 19(8): 2211, doi: 10.3390/ijms19082211.
37.
Wang Y., Koh W.P., Jensen M.K., Yuan J.M., Pan A. Plasma fetuin-A levels and risk of type 2 diabetes mellitus in a Chinese population: a nested case-control study. Diabetes Metab. J. 2019; 43(4): 474–486, doi: 10.4093/dmj.2018.0171.
38.
Ricken F., Can A.D., Gräber S., Häusler M., Jahnen-Dechent W. Post-translational modifications glycosylation and phosphorylation of the major hepatic plasma protein fetuin-A are associated with CNS inflammation in children. PLoS One 2022; 17(10): e0268592, doi: 10.1371/journal.pone.0268592.
39.
Harbuwono D.S., Sazli B.I., Kurniawan F., Darmowidjojo B., Koesnoe S., Tahapary D.L. The impact of Ramadan fasting on fetuin-A level in type 2 diabetes mellitus. Heliyon 2021; 7(5): e06773, doi: 10.1016/j.heliyon.2021.e06773.
40.
Roshanzamir F., Miraghajani M., Rouhani M.H., Mansourian M., Ghiasvand R., Safavi S.M. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J. Endocrinol. Invest. 2018; 41(1): 33–47, doi: 10.1007/s40618-017-0697-8.
41.
Guo V.Y., Cao B., Cai C., Cheng K.K., Cheung B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2018; 55(1): 87–98, doi: 10.1007/s00592-017-1068-9.
42.
Robinson K.N., Teran-Garcia M. From infancy to aging: Biological and behavioral modifiers of fetuin-A. Biochimie 2016; 124: 141–149, doi: 10.1016/j.biochi.2015.12.016.
43.
Chekol Abebe E., Tilahun Muche Z., Behaile T/Mariam A., Mengie Ayele T., Mekonnen Agidew M., Teshome Azezew M. et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front. Cell Dev. Biol. 2022; 10: 945287, doi: 10.3389/fcell.2022.945287.
44.
Selvaraju V., Babu J.R., Geetha T. Multiplexed measurements of salivary fetuin-A, insulin, and adiponectin as potential non-invasive biomarkers in childhood obesity. Cytokine 2022; 153: 155843, doi: 10.1016/j.cyto.2022.155843.
45.
Sujana C., Huth C., Zierer A., Meesters S., Sudduth-Klinger J., Koenig W. et al. Association of fetuin-A with incident type 2 diabetes: results from the MONICA/KORA Augsburg study and a systematic meta-analysis. Eur. J. Endocrinol. 2018; 178(4): 389–398, doi: 10.1530/EJE-17-1053.
46.
Kim J.E., Kim J.S., Jo M.J., Cho E., Ahn S.Y., Kwon Y.J. et al. The roles and associated mechanisms of adipokines in development of metabolic syndrome. Molecules 2022; 27(2): 334, doi: 10.3390/molecules27020334.
47.
Bourebaba L., Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J. Clin. Med. 2019; 8(12): 2033, doi: 10.3390/jcm8122033.
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.