Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Kleszczowe zapalenie mózgu i diagnostyka różnicowa
1
Studenckie Koło Naukowe przy Oddziale Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, Polska / Students’ Scientific Club, Department of Pediatrics and Developmental Neurology, Upper Silesian Child Health Center named after John Paul II, Independent Public Clinical Hospital No. 6 of the Medical University of Silesia, Katowice, Poland
2
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, Polska / Department of Pediatrics and Developmental Neurology, Upper Silesian Child Health Center named after John Paul II, Independent Public Clinical Hospital No. 6 of the Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Patrycja Ochman-Pasierbek
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Ann. Acad. Med. Siles. 2024;78:234-247
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie:
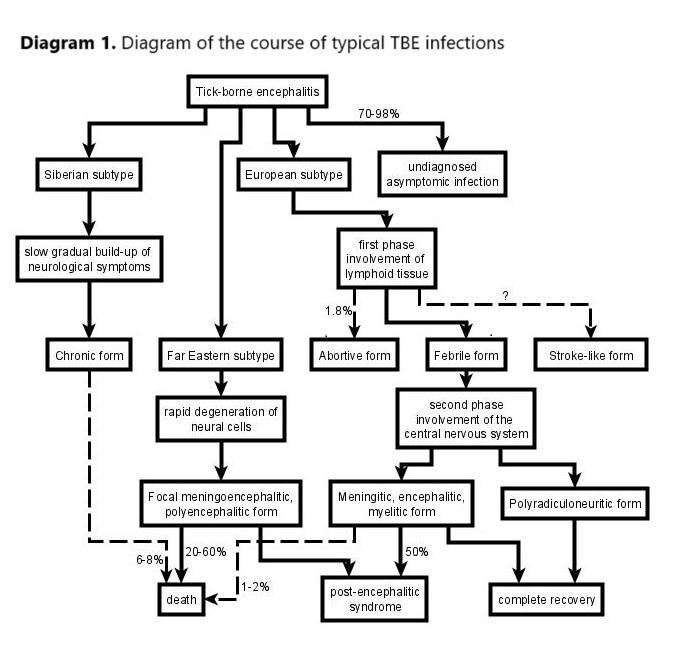
Kleszczowe zapalenie mózgu (tick-borne encephalitis – TBE) jest chorobą wywoływaną przez wirusa kleszczowego zapalenia mózgu, który jest przenoszony przez ukąszenia kleszczy. Rozpowszechnienie TBE ocenia się na 10,000–15,000 przypadków rocznie i jest porównywalne dla krajów europejskich i azjatyckich. Około 10–20% wszystkich zainfekowanych osób jest w wieku dziecięcym. Znaczna większość, bo aż 70–98% z nich, przechodzi tę chorobę bezobjawowo lub jest niezdiagnozowana. Do głównych objawów klinicznych zalicza się zapalenie opon mózgowo-rdzeniowych (obecne w 69% przypadków) i mózgu (30%) oraz rdzenia kręgowego (1%). U około 2,1% pacjentów rozwijają się długotrwałe następstwa neurologiczne.
Metodyka:
Artykuły do pracy zostały wybrane z trzech ogólnodostępnych baz danych. Wykorzystano w tym celu następujące hasła: „infection/epidemiology” + „tick bites/tick-borne encephalitis” + „clinical manifestation/pathogenesis/treatment” oraz „aseptic/viral/bacterial” + „encephalitis/meningitis”. Ostatecznie wybrano 71 prac naukowych i 8 witryn internetowych opublikowanych w latach 1995–2023.
Stan wiedzy:
TBE, ze względu na wektor kleszczowy, może być różnicowane z takimi jednostkami chorobowymi jak: babeszjoza, borelioza, południowa wysypka związana z kleszczami (southern tick-associated rash illness – STARI), chlamydioza, erlichioza, gorączka kleszczowa Kolorado (Colorado tick fever – CTF), wirus Heartland (HRTV), wirus Powassan (POWV), anaplazmoza granulocytarna, dur powrotny (tick-borne relapsing fever – TBRF), toksoplazmoza, tularemia, riketsjozy; lub z jednostkami o podobnej symptomatologii, takimi jak: udar mózgu, bruceloza, mononukleoza zakaźna (infectious mononucleosis – IM), żółta febra (yellow fever – YF), japońskie zapalenie mózgu (Japanese encephalitis – JE), inne wirusowe zapalenie opon mózgowo-rdzeniowych, mózgu, rdzenia kręgowego oraz aseptyczne zapalenie opon mózgowo-rdzeniowych.
Wnioski:
Diagnostyka różnicowa TBE jest obszerna i powinna obejmować szeroki zakres zakażeń ośrodkowego układu nerwowego wywołanych zarówno przez inne czynniki zakaźne, jak i choroby niezakaźne.
Kleszczowe zapalenie mózgu (tick-borne encephalitis – TBE) jest chorobą wywoływaną przez wirusa kleszczowego zapalenia mózgu, który jest przenoszony przez ukąszenia kleszczy. Rozpowszechnienie TBE ocenia się na 10,000–15,000 przypadków rocznie i jest porównywalne dla krajów europejskich i azjatyckich. Około 10–20% wszystkich zainfekowanych osób jest w wieku dziecięcym. Znaczna większość, bo aż 70–98% z nich, przechodzi tę chorobę bezobjawowo lub jest niezdiagnozowana. Do głównych objawów klinicznych zalicza się zapalenie opon mózgowo-rdzeniowych (obecne w 69% przypadków) i mózgu (30%) oraz rdzenia kręgowego (1%). U około 2,1% pacjentów rozwijają się długotrwałe następstwa neurologiczne.
Metodyka:
Artykuły do pracy zostały wybrane z trzech ogólnodostępnych baz danych. Wykorzystano w tym celu następujące hasła: „infection/epidemiology” + „tick bites/tick-borne encephalitis” + „clinical manifestation/pathogenesis/treatment” oraz „aseptic/viral/bacterial” + „encephalitis/meningitis”. Ostatecznie wybrano 71 prac naukowych i 8 witryn internetowych opublikowanych w latach 1995–2023.
Stan wiedzy:
TBE, ze względu na wektor kleszczowy, może być różnicowane z takimi jednostkami chorobowymi jak: babeszjoza, borelioza, południowa wysypka związana z kleszczami (southern tick-associated rash illness – STARI), chlamydioza, erlichioza, gorączka kleszczowa Kolorado (Colorado tick fever – CTF), wirus Heartland (HRTV), wirus Powassan (POWV), anaplazmoza granulocytarna, dur powrotny (tick-borne relapsing fever – TBRF), toksoplazmoza, tularemia, riketsjozy; lub z jednostkami o podobnej symptomatologii, takimi jak: udar mózgu, bruceloza, mononukleoza zakaźna (infectious mononucleosis – IM), żółta febra (yellow fever – YF), japońskie zapalenie mózgu (Japanese encephalitis – JE), inne wirusowe zapalenie opon mózgowo-rdzeniowych, mózgu, rdzenia kręgowego oraz aseptyczne zapalenie opon mózgowo-rdzeniowych.
Wnioski:
Diagnostyka różnicowa TBE jest obszerna i powinna obejmować szeroki zakres zakażeń ośrodkowego układu nerwowego wywołanych zarówno przez inne czynniki zakaźne, jak i choroby niezakaźne.
REFERENCJE (79)
1.
Bogovic P., Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015; 3(5): 430–441, doi: 10.12998/wjcc.v3.i5.430.
2.
Gritsun T.S., Lashkevich V.A., Gould E.A. Tick-borne encephalitis. Antiviral Res. 2003; 57(1–2): 129–146, doi: 10.1016/S0166-3542(02)00206-1.
3.
Kmieciak W., Ciszewski M., Szewczyk E.M. Tick-borne diseases in Poland: Prevalence and difficulties in diagnostics. [Article in Polish]. Med. Pr. 2016; 67(1): 73–87, doi: 10.13075/mp.5893.00264.
4.
Zajkowska J., Waluk E., Dunaj J., Świerzbińska R., Hordowicz M., Zajkowska O. et al. Assessment of the potential effect of the implementation of serological testing tick-borne encephalitis on the detection of this disease on areas considered as non-endemic in Poland – preliminary report. Przegl. Epidemiol. 2021; 75(4): 515–523, doi: 10.32394/pe.75.48.
5.
Stragapede L., Dinoto A., Cheli M., Manganotti P. Epilepsia partialis continua following a Western variant tick-borne encephalitis. J. Neurovirol. 2018; 24(6): 773–775, doi: 10.1007/s13365-018-0671-z.
6.
Eggers C., Burghaus L., Fink G.R., Dohmen C. Epilepsia partialis continua responsive to intravenous levetiracetam. Seizure 2009; 18(10): 716–718, doi: 10.1016/j.seizure.2009.09.005.
7.
Mameniškienė R., Wolf P. Epilepsia partialis continua: A review. Seizure 2017; 44: 74–80, doi: 10.1016/j.seizure.2016.10.010.
8.
Motika P.V., Bergen D.C. Epilepsia Partialis Continua. In: Encyclopedia of Movement Disorders. K. Kompoliti, L.V. Metman [ed.]. Academic Press, 2010, p. 450–452, doi: 10.1016/B978-0-12-374105-9.00028-9.
9.
Gritsun T.S., Nuttall P.A., Gould E.A. Tick-borne flaviviruses. Adv. Virus Res. 2003; 61: 317–371, doi: 10.1016/s0065-3527(03)61008-0.
10.
Beltz L.A. Zika and Other Neglected and Emerging Flaviviruses: The Continuing Threat to Human Health. Elsevier 2021.
11.
Růžek D., Dobler G., Mantke O.D. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010; 8(4): 223–232, doi: 10.1016/j.tmaid.2010.06.004.
12.
Eyer L., Seley-Radtke K., Ruzek D. New directions in the experimental therapy of tick-borne encephalitis. Antiviral Res. 2023; 210: 105504, doi: 10.1016/j.antiviral.2022.105504.
13.
Rostasy K. Tick-borne encephalitis in children. Wien. Med. Wochenschr. 2012; 162(11–12): 244–247, doi: 10.1007/s10354-012-0101-4.
14.
Pancewicz S.A., Hermanowska-Szpakowicz T., Kondrusik M., Zajkowska J.M., Grygorczuk S., Świerzbińska R. Aspekty epidemiologiczno-kliniczne i profilaktyka kleszczowego zapalenia mózgu. Pol. Przegl. Neurol. 2006; 2(1): 7–12.
15.
Ruzek D., Avšič Županc T., Borde J., Chrdle A., Eyer L., Karganova G. et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 2019; 164: 23–51, doi: 10.1016/j.antiviral.2019.01.014.
16.
Lindquist L., Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371(9627): 1861–1871, doi: 10.1016/S0140-6736(08)60800-4.
17.
Riccardi N., Antonello R.M., Luzzati R., Zajkowska J., Di Bella S., Giacobbe D.R. Tick-borne encephalitis in Europe: a brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019; 62: 1–6, doi: 10.1016/j.ejim.2019.01.004.
18.
Pulkkinen L.I.A., Butcher S.J., Anastasina M. Tick-borne encephalitis virus: A structural view. Viruses 2018; 10(7): 350, doi: 10.3390/v10070350.
19.
Beauté J., Spiteri G., Warns-Petit E., Zeller H. Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill. 2018; 23(45): 1800201, doi: 10.2807/1560-7917.ES.2018.23.45.1800201.
20.
Amicizia D., Domnich A., Panatto D., Lai P.L., Cristina M.L., Avio U. et al. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccin. Immunother. 2013; 9(5): 1163–1171, doi: 10.4161/hv.23802.
21.
Kuchar E., Zajkowska J., Flisiak R., Mastalerz-Migas A., Rosińska M., Szenborn L. et al. Epidemiology, diagnosis, and prevention of tick-borne encephalitis in Poland and selected European countries – a position statement of the Polish group of experts. [Article in Polish]. Med. Pr. 2021; 72(2): 193–210, doi: 10.13075/mp.5893.01063.
22.
European Centre for Disease Prevention and Control. Tick-borne encephalitis. In: ECDC. Annual epidemiological report for 2020. Stockholm: ECDC; 2022.
23.
Sapi E., Gupta K., Wawrzeniak K., Gaur G., Torres J., Filush K. et al. Borrelia and Chlamydia can form mixed biofilms in infected human skin tissues. Eur. J. Microbiol. Immunol. (Bp) 2019; 9(2): 46–55, doi: 10.1556/1886.2019.00003.
24.
Davar K., Wilson M.R., Miller S., Chiu C.Y., Vijayan T. A rare bird: Diagnosis of psittacosis meningitis by clinical metagenomic next-generation sequencing. Open Forum Infect. Dis. 2021; 8(12): ofab555; doi: 10.1093/ofid/ofab555.
25.
Dard C., Fricker-Hidalgo H., Brenier-Pinchart M.P., Pelloux H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 2016; 32(6): 492–506, doi: 10.1016/j.pt.2016.04.001.
26.
Ben-Harari R.R. Tick transmission of toxoplasmosis. Expert Rev. Anti Infect. Ther. 2019; 17(11): 911–917, doi: 10.1080/14787210.2019.1682550.
27.
Rawlings J.A. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex. Med. 1995; 91(5): 56–59.
28.
Furtado J.M., Smith J.R., Belfort R. Jr, Gattey D., Winthrop K.L. Toxoplasmosis: a global threat. J. Glob. Infect. Dis. 2011; 3(3): 281–284, doi: 10.4103/0974-777X.83536.
29.
Weiss L.M., Dubey J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009; 39(8): 895–901, doi: 10.1016/j.ijpara.2009.02.004.
30.
Elsheikha H.M., Marra C.M., Zhu X.Q. Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin. Microbiol. Rev. 2020; 34(1): e00115–00119, doi: 10.1128/CMR.00115-19.
31.
Fuglewicz A.J., Piotrowski P., Stodolak A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017; 26(6): 1031–1036, doi: 10.17219/acem/61435.
32.
Schoen R.T. Lyme disease: diagnosis and treatment. Curr. Opin. Rheumatol. 2020; 32(3): 247–254, doi: 10.1097/BOR.0000000000000698.
33.
Abdad M.Y., Abou Abdallah R., Fournier P.E., Stenos J., Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018; 56(8): e01728-17, doi: 10.1128/JCM.01728-17.
34.
Jorge Miranda R., Salim Mattar V., Marco Gonzalez T. Rickettsiosis. Rev. MVZ Cordoba 2017; 22(Supl): 6118–6133, doi: 10.21897/rmvz.1080.
35.
Corrin T., Greig J., Harding S., Young I., Mascarenhas M., Waddell L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018; 65(6): 595–624, doi: 10.1111/zph.12485.
36.
Bakken J.S., Dumler J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 2015; 29(2): 341–355, doi: 10.1016/j.idc.2015.02.007.
37.
Factsheet on Human granulocytic anaplasmosis. European Centre for Disease Prevention and Control [online] https://web.archive.org/web/20... [accessed on 6 June 2022].
38.
Cho J.M., Chang J., Kim D.M., Kwak Y.G., Cho C.R., Song J.E. Human granulocytic anaplasmosis combined with rhabdomyolysis: a case report. BMC Infect. Dis. 2021; 21(1): 1184, doi: 10.1186/s12879-021-06869-z.
39.
Kandhi S., Ghazanfar H., Qureshi Z.A., Kalangi H., Jyala A., Arguello Perez E.S. An atypical presentation of a severe case of anaplasma phagocytophilum. Cureus 2022; 14(3): e23224, doi: 10.7759/cureus.23224.
40.
de Jesus M., Lopez A., Yabut J., Vu S., Manne M., Ibrahim L., Mutneja R. Anaplasmosis-induced hemophagocytic lymphohistiocytosis. Proc. (Bayl. Univ. Med. Cent.) 2022; 35(3): 379–381, doi: 10.1080/08998280.2022.2039046.
41.
Abdelmaseih R., Ashraf B., Abdelmasih R., Dunn S., Nasser H. Southern tick-associated rash illness: Florida’s Lyme disease variant. Cureus 2021; 13(5): e15306, doi: 10.7759/cureus.15306.
42.
Nualnoi T., Kirosingh A., Basallo K., Hau D., Gates-Hollingsworth M.A., Thorkildson P. et al. Immunoglobulin G subclass switching impacts sensitivity of an immunoassay targeting Francisella tularensis lipopolysaccharide. PLoS One 2018; 13(4): e0195308, doi: 10.1371/journal.pone.0195308.
43.
Faber M., Heuner K., Jacob D., Grunow R. Tularemia in Germany – A re-emerging zoonosis. Front. Cell. Infect. Microbiol. 2018; 8: 40, doi: 10.3389/fcimb.2018.00040.
44.
Rochlin I., Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 2020; 69(6): 781–791, doi: 10.1099/jmm.0.001206.
45.
Biggs H.M., Behravesh C.B., Bradley K.K., Dahlgren F.S., Drexler N.A., Dumler J.S, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis – United States. MMWR Recomm. Rep. 2016; 65(2): 1–44, doi: 10.15585/mmwr.rr6502a1.
46.
Heartland Virus. Columbia University Irving Medical Center [online] https://www.columbia-lyme.org/... [accessed on 6 June 2022].
47.
Staples J.E., Pastula D.M., Panella A.J., Rabe I.B., Kosoy O.I., Walker W.L. et al. Investigation of Heartland virus disease throughout the United States, 2013–2017. Open Forum Infect. Dis. 2020; 7(5): ofaa125, doi: 10.1093/ofid/ofaa125.
48.
Brault A.C., Savage H.M., Duggal N.K., Eisen R.J., Staples J.E. Heartland virus epidemiology, vector association, and disease potential. Viruses 2018; 10(9): 498, doi: 10.3390/v10090498.
49.
Tuten H.C., Burkhalter K.L., Noel K.R., Hernandez E.J., Yates S., Wojnowski K. et al. Heartland virus in humans and ticks, Illinois, USA, 2018–2019. Emerg. Infect. Dis. 2020; 26(7): 1548–1552, doi: 10.3201/eid2607.200110.
50.
Pastula D.M., Turabelidze G., Yates K.F., Jones T.F., Lambert A.J., Panella A.J. et al. Notes from the field: Heartland virus disease – United States, 2012–2013. MMWR Morb. Mortal. Wkly Rep. 2014; 63(12): 270–271.
51.
Jakab Á., Kahlig P., Kuenzli E., Neumayr A. Tick borne relapsing fever – a systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 2022; 16(2): e0010212, doi: 10.1371/journal.pntd.0010212.
52.
Domínguez M.C., Vergara S., Gómez M.C., Roldán M.E. Epidemiology of tick-borne relapsing fever in endemic area, Spain. Emerg. Infect. Dis. 2020; 26(5): 849–856, doi: 10.3201/eid2605.190745.
53.
Tick and Louse-borne Relapsing Fevers. CDC [online] https://www.cdc.gov/relapsing-... [accessed on 6 June 2022].
55.
Ebel G.D. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol. 2010; 55: 95–110, doi: 10.1146/annurev-ento-112408-085446.
56.
Williamson B.N., Fischer R.J., Lopez J.E., Ebihara H., Schwan T.G. Prevalence and strains of Colorado tick fever virus in Rocky Mountain wood ticks in the Bitterroot Valley, Montana. Vector Borne Zoonotic Dis. 2019; 19(9): 694–702, doi: 10.1089/vbz.2018.2407.
57.
Padgett K.A., Kjemtrup A., Novak M., Velez J.O., Panella N. Colorado tick fever virus in the Far West: forgotten, but not gone. Vector Borne Zoonotic Dis. 2022; 22(8): 443–448, doi: 10.1089/vbz.2022.0018.
58.
Pecina C.A. Tick paralysis. Semin. Neurol. 2012; 32(5): 531–532, doi: 10.1055/s-0033-1334474.
59.
Molins C.R., Ashton L.V., Wormser G.P., Andre B.G., Hess A.M., Delorey M.J. et al. Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci. Transl. Med. 2017; 9(403): eaal2717, doi: 10.1126/scitranslmed.aal2717.
60.
Seo J.W., Kim D., Yun N., Kim D.M. Clinical update of severe fever with thrombocytopenia syndrome. Viruses 2021; 13(7): 1213, doi: 10.3390/v13071213.
61.
Moniuszko A., Dunaj J., Czupryna P., Zajkowska J., Pancewicz S. Neoehrlichiosis – a new tick-borne disease – is there a threat in Poland? Przegl. Epidemiol. 2015; 69(1): 23–26, 131–133.
62.
Tyrakowska-Dadełło Z., Tarasów E., Janusek D., Moniuszko-Malinowska A., Zajkowska J., Pancewicz S. Brain perfusion alterations in tick-borne encephalitis-preliminary report. Int. J. Infect. Dis. 2018; 68: 26–30, doi: 10.1016/j.ijid.2018.01.002.
63.
Eleftheriou A., Lundin F., Petropoulos E.A. Tick-borne encephalitis: stroke-like presentation. J. Stroke Cerebrovasc. Dis. 2019; 28(8): e119–e122, doi: 10.1016/j.jstrokecerebrovasdis.2019.05.028.
64.
Tarfarosh S.F., Manzoor M. Neurological manifestations of Brucellosis in an Indian population. Cureus 2016; 8(7): e684, doi: 10.7759/cureus.684.
65.
Bukhari E.E. Pediatric brucellosis: An update review for the new millennium. Saudi Med. J. 2018; 39(4): 336–341, doi: 10.15537/smj.2018.4.21896.
66.
Choroby zakaźne i zatrucia w Polsce rok 2017 (Tabele). Narodowy Instytut Zdrowia Publicznego – Państwowy Instytut Badawczy [online] https://epibaza.pzh.gov.pl/cho... [accessed on 24 January 2024].
67.
Cai X., Ebell M.H., Haines L. Accuracy of signs, symptoms, and hematologic parameters for the diagnosis of infectious mononucleosis: A systematic review and meta-analysis. J. Am. Board Fam. Med. 2021; 34(6): 1141–1156, doi: 10.3122/jabfm.2021.06.210217.
68.
Arslan F., Karagöz E., Beköz H.S., Ceylan B., Mert A. Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis presenting with acute sensorineural hearing loss: a case report and review of the literature. Infez. Med. 2017; 25(3): 277–280.
69.
Simon L.V., Hashmi M.F., Torp K.D. Yellow fever. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan, https://www.ncbi.nlm.nih.gov/b... [accessed on 6 June 6 2022].
70.
Douam F., Ploss A. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018; 26(11): 913–928, doi: 10.1016/j.tim.2018.05.012.
71.
Yellow fever. World Health Organization, 31 May 2023 [online] https://www.who.int/news-room/... [accessed on 6 June 2022].
72.
Tattevin P., Tchamgoué S., Belem A., Bénézit F., Pronier C., Revest M. Aseptic meningitis. Rev. Neurol. (Paris) 2019; 175(7–8): 475–480, doi: 10.1016/j.neurol.2019.07.005.
73.
Wright W.F., Pinto C.N., Palisoc K., Baghli S. Viral (aseptic) meningitis: A review. J. Neurol. Sci. 2019; 398: 176–183, doi: 10.1016/j.jns.2019.01.050.
74.
Clark M.B., Schaefer T.J. West Nile virus. 2022 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan, https://www.ncbi.nlm.nih.gov/b... [accessed on 6 June 2022].
75.
Shin A., Tukhanova N., Ndenkeh J. Jr, Shapiyeva Z., Yegemberdiyeva R., Yeraliyeva L. et al. Tick-borne encephalitis virus and West-Nile fever virus as causes of serous meningitis of unknown origin in Kazakhstan. Zoonoses Public Health 2022; 69(5): 514–525, doi: 10.1111/zph.12941.
76.
Schwarz L., Akbari N., Prüss H., Meisel A., Scheibe F. Clinical characteristics, treatments, outcome, and prognostic factors of severe autoimmune encephalitis in the intensive care unit: Standard treatment and the value of additional plasma cell-depleting escalation therapies for treatment‐refractory patients. Eur. J. Neurol. 2023; 30(2): 474–489, doi: 10.1111/ene.15585.
77.
Machado S., Pinto A.N., Irani S.R. What should you know about limbic encephalitis? Arq. Neuropsiquiatr. 2012; 70(10): 817–822, doi: 10.1590/S0004-282x2012001000012.
78.
Lotric-Furlan S., Avsic-Zupanc T., Strle F. An abortive form of tick-borne encephalitis (TBE): a rare clinical manifestation of infection with TBE virus. Wien. Klin. Wochenschr. 2002; 114(13–14): 627–629.
79.
Logina I., Krumina A., Karelis G., Elsone L., Viksna L., Rozentale B. et al. Clinical features of double infection with tick-borne encephalitis and Lyme borreliosis transmitted by tick bite. J. Neurol. Neurosurg. Psychiatry 2006; 77(12): 1350–1353, doi: 10.1136/jnnp.2004.060731.
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.