Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Wybrane adipokiny jako potencjalne czynniki prognostyczne i diagnostyczne
w leczeniu zaburzeń metabolicznych towarzyszących otyłości
1
Students’ Scientific Club, Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Students’ Scientific Club, Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
4
Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Klaudia Stocerz
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Studenckie Koło Naukowe przy Zakładzie Farmacji Aptecznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jedności 10, 41-205 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:138-145
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
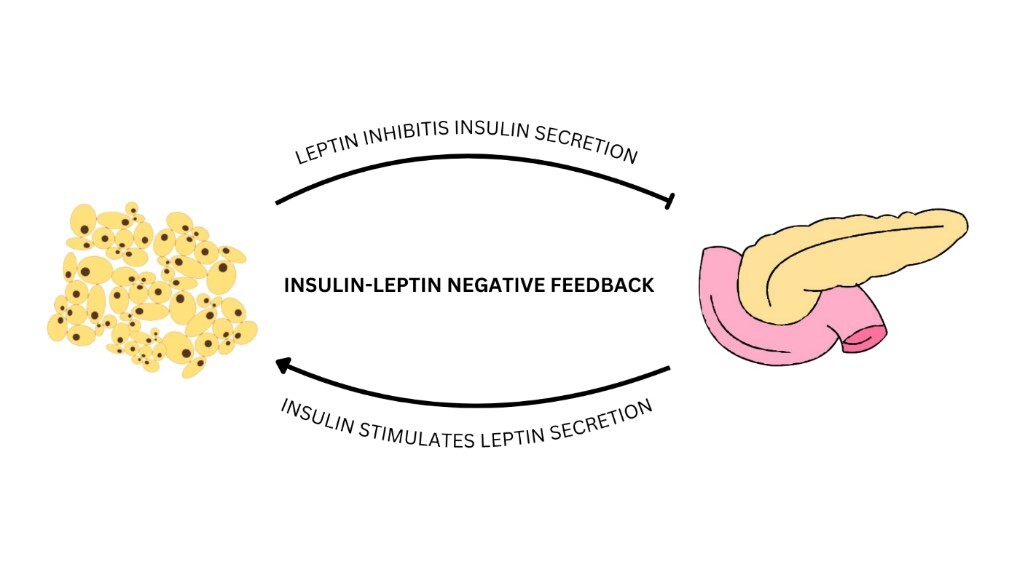
Otyłość to przewlekła choroba, stanowiąca globalny problem i poważne wyzwanie dla zdrowia publicznego. W jej przebiegu dochodzi do nadmiernego nagromadzenia się tkanki tłuszczowej w organizmie, co prowadzi nie tylko do wzrostu ryzyka wystąpienia powikłań zdrowotnych, lecz także negatywnie wpływa na jakość życia. Komórki tłuszczowe – adipocyty – są odpowiedzialne za biosyntezę i uwalnianie adipokin, do których należą m.in. leptyna, wisfatyna, chemeryna oraz omentyna-1. Są to aktywne substancje biologiczne, mogące wykazywać właściwości prozapalne lub przeciwzapalne. Zaburzenie równowagi pomiędzy adipokinami prozapalnymi i adipokinami przeciwzapalnymi może prowadzić do rozwoju zaburzeń metabolicznych. Wspomniane właściwości adipokin sprawiają, że możliwe jest ich potencjalne wykorzystanie jako czynników diagnostycznych oraz terapeutycznych w przebiegu otyłości, a także towarzyszących jej zaburzeń, takich jak insulinooporność, cukrzyca typu 2 czy powikłania kardiometaboliczne. Pomimo znacznego postępu w poznaniu roli, jaką odgrywają omówione adipokiny, konieczne są dalsze badania, których wyniki umożliwią precyzyjne omówienie mechanizmów ich działania oraz określą ścisłe zależności ich stężeń w osoczu od stanu choroby.
REFERENCJE (52)
1.
Zatterale F., Longo M., Naderi J., Raciti G.A., Desiderio A., Miele C., Beguinot F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020; 10: 1607, doi: 10.3389/fphys.2019.01607.
2.
Longo M., Spinelli R., D’Esposito V., Zatterale F., Fiory F., Nigro C. et al. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim. Biophys. Acta 2016; 1863(6 Pt A): 1146–1156, doi: 10.1016/j.bbamcr.2016.02.019.
3.
Chen L., Chen R., Wang H., Liang F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015; 2015: 508409, doi: 10.1155/2015/508409.
4.
Ahmed B., Sultana R., Greene M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021; 137: 111315, doi: 10.1016/j.biopha.2021.111315.
5.
Ormazábal V., Nair S., Elfeky O., Aguayo C., Salomón C., Zúñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018; 17(1): 122, doi: 10.1186/s12933-018-0762-4.
6.
Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019; 234(6): 8152–8161, doi: 10.1002/jcp.27603.
7.
Li M., Chi X., Wang Y., Setrerrahmane S., Xie W., Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022; 7(1): 216, doi: 10.1038/s41392-022-01073-0.
8.
Sangwung P., Petersen K.F., Shulman G.I., Knowles J.W. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology 2020; 161(4): bqaa017, doi: 10.1210/endocr/bqaa017.
9.
Araszkiewicz A., Bandurska-Stankiewicz E., Borys S., Budzyński A., Cyganek K., Cypryk K. et al. 2023 Guidelines on the management of patients with diabetes – A position of Diabetes Poland. Curr. Top. Diabetes 2023; 3(1): 1–133, doi: 10.5114/ctd/160061.
10.
Harreiter J., Roden M. Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023) [Article in German]. Wien. Klin. Wochenschr. 2023; 135(Suppl 1): 7–17, doi: 10.1007/s00508-022-02122-y.
11.
Alam S., Hasan MdK., Neaz S., Hussain N., Hossain MdF., Rahman T. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021; 2(2): 36–50, doi: 10.3390/diabetology2020004.
12.
Liang W., Ye D.D. The potential of adipokines as biomarkers and therapeutic agents for vascular complications in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2019; 48: 32–39, doi: 10.1016/j.cytogfr.2019.06.002.
13.
Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch. Med. Sci. 2013; 9(2): 191–200, doi: 10.5114/aoms.2013.33181.
14.
Taylor E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021; 135(6): 731–752, doi: 10.1042/cs20200895.
15.
Obradović M., Sudar-Milovanović E., Šoškić S., Essack M., Arya S., Stewart A.J. et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. 2021; 12: 585887, doi: 10.3389/fendo.2021.585887.
16.
Coppari R., Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 2012; 11(9): 692–708, doi: 10.1038/nrd3757.
17.
Meek T.H., Morton G.J. The role of leptin in diabetes: metabolic effects. Diabetologia 2016; 59(5): 928–932, doi: 10.1007/s00125-016-3898-3.
18.
Moonishaa T.M., Nanda S.K., Shamraj M., Sivaa R., Sivakumar P., Ravichandran K. Evaluation of leptin as a marker of insulin resistance in type 2 diabetes mellitus. Int. J. Appl. Basic Med. Res. 2017; 7(3): 176–180, doi: 10.4103/ijabmr.ijabmr_278_16.
19.
Katsiki N., Mikhailidis D.P., Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018; 39(7): 1176–1188, doi: 10.1038/aps.2018.40.
20.
Kurajoh M., Koyama H., Kadoya M., Naka M., Miyoshi A., Kanzaki A. et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc. Diabetol. 2015; 14: 117, doi: 10.1186/s12933-015-0280-6.
21.
Morioka T., Emoto M., Yamazaki Y., Kawano N., Imamura S., Numaguchi R. et al. Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc. Diabetol. 2014; 13: 10, doi: 10.1186/1475-2840-13-10.
22.
Paz-Filho G., Mastronardi C., Wong M.L., Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 2012; 16(Suppl 3): S549–S555, doi: 10.4103/2230-8210.105571.
23.
Jung C.H., Kim B.Y., Mok J.O., Kang S.K., Kim C.H. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014; 5(3): 333–339, doi: 10.1111/jdi.12144.
24.
Vavruch C., Länne T., Fredrikson M., Lindström T., Östgren C.J., Nyström F.H. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc. Diabetol. 2015; 14: 62, doi: 10.1186/s12933-015-0208-1.
25.
El Husseny M.W., Mamdouh M., Shaban S., Ibrahim Abushouk A., Zaki M.M., Ahmed O.M. et al. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J. Diabetes Res. 2017; 2017: 8095926, doi: 10.1155/2017/8095926.
26.
Frühbeck G., Catalán V., Rodrı́guez A., Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018; 7(1): 57–62, doi: 10.1080/21623945.2017.1402151.
27.
Farr O.M., Gavrieli A., Mantzoros C.S. Leptin applications in 2015: what have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015; 22(5): 353–359, doi: 10.1097/med.0000000000000184.
28.
Zhao S., Zhu Y., Schultz R.D., Li N., He Z., Zhang Z. et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 2019; 30(4): 706–719.e6, doi: 10.1016/j.cmet.2019.08.005.
29.
Dakroub A., Nasser S.A., Younis N., Bhagani H., Al-Dhaheri Y., Pintus G. et al. Visfatin: a possible role in cardiovasculo-metabolic disorders. Cells 2020; 9(11): 2444, doi: 10.3390/cells9112444.
30.
Abdalla M.M.I. Role of visfatin in obesity-induced insulin resistance. World J. Clin. Cases 2022; 10(30): 10840–10851, doi: 10.12998/wjcc.v10.i30.10840.
31.
Hognogi L.D., Simiti L.V. The cardiovascular impact of visfatin – an inflammation predictor biomarker in metabolic syndrome. Clujul Med. 2016; 89(3): 322–326, doi: 10.15386/cjmed-591.
32.
Zheng L.Y., Xu X., Wan R.H., Sheng X., Lu J., Huang Q. Association between serum visfatin levels and atherosclerotic plaque in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2019; 11: 60, doi: 10.1186/s13098-019-0455-5.
33.
Uslu S., Kebapçı N., Kara M., Bal C. Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2012; 4(1): 113–120, doi: 10.3892/etm.2012.557.
34.
Ali S., Alam R., Ahsan H., Khan S. Role of adipokines (omentin and visfatin) in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2023; 33(3): 483–493, doi: 10.1016/j.numecd.2022.11.023.
35.
Léniz A., González M., Besné I., Carr-Ugarte H., Gómez-García I., Portillo M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022; 541: 111504, doi: 10.1016/j.mce.2021.111504.
36.
Fatima S.S., Butt Z., Bader N., Pathan A.Z., Hussain S., Iqbal N.T. Role of multifunctional Chemerin in obesity and preclinical diabetes. Obes. Res. Clin. Pract. 2015; 9(5): 507–512, doi: 10.1016/j.orcp.2015.01.004.
37.
Fatima S.S., Alam F., Chaudhry B., Khan T.A. Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2017; 30(9): 1023–1028, doi: 10.1080/14767058.2016.1199671.
38.
Zhou Z., Chen H., Ju H., Sun M. Circulating chemerin levels and gestational diabetes mellitus: a systematic review and meta-analysis. Lipids Health Dis. 2018; 17(1): 169, doi: 10.1186/s12944-018-0826-1.
39.
Yu S., Zhang Y., Li M.Z., Xu H., Wang Q., Song J. et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin. Med. J. (Engl) 2012; 125(19): 3440–3444, doi: 10.3760/cma.j.issn.0366-6999.2012.19.015.
40.
Mir M.M., Mir R., Alghamdi M.A.A., Wani J.I., Sabah Z.U., Jeelani M. et al. Differential association of selected adipocytokines, adiponectin, leptin, resistin, visfatin and chemerin, with the pathogenesis and progression of type 2 diabetes mellitus (T2DM) in the Asir region of Saudi Arabia: A case control study. J. Pers. Med. 2022; 12(5): 735, doi: 10.3390/jpm12050735.
41.
Waluga-Kozlowska E., Kuznik-Trocha K., Komosinska-Vassev K., Olczyk P., Jura-Poltorak A., Winsz-Szczotka K. et al. Progranulin and chemerin plasma level in obese patients with type 2 diabetes treated with a long-acting insulin analogue and premixed insulin analogue. J. Physiol. Pharmacol. 2021; 72(6), doi: 10.26402/jpp.2021.6.07.
42.
Neuparth M.J., Proença J.B., Santos‐Silva A., Coimbra S. The positive effect of moderate walking exercise on chemerin levels in Portuguese patients with type 2 diabetes mellitus. J. Investig. Med. 2014; 62(2): 350–353, doi: 10.2310/JIM.0000000000000025.
43.
Bobbert T., Schwärz F., Fischer-Rosinský A., Maurer L., Möhlig M., Pfeiffer A.F.H. et al. Chemerin and prediction of Diabetes mellitus type 2. Clin. Endocrinol. 2015; 82(6): 838–843, doi: 10.1111/cen.12707.
44.
Tan L., Lu X., Danser A.H.J., Verdonk K. The role of chemerin in metabolic and cardiovascular disease: A literature review of its physiology and pathology from a nutritional perspective. Nutrients 2023; 15(13): 2878, doi: 10.3390/nu15132878.
45.
Mačvanin M.T., Rizzo M., Radovanović J., Sönmez A., Paneni F., Isenović E.R. Role of chemerin in cardiovascular diseases. Biomedicines 2022; 10(11): 2970, doi: 10.3390/biomedicines10112970.
46.
Waluga-Kozłowska E., Komosińska-Vassev K., Szczepański J., Olczyk P. Omentyna – nowy biomarker w medycynie? Część 1. Farm. Pol. 2018; 74(9): 535–541.
47.
Elsaid N.H., Sadik N.A., Ahmed N.R., Fayez S.E., Mohammed N.A.E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J. Clin. Transl. Endocrinol. 2018; 13: 14–19, doi: 10.1016/j.jcte.2018.05.003.
48.
Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C. et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006; 290(6): E1253–1261, doi: 10.1152/ajpendo.00572.2004.
49.
Pan X., Kaminga A.C., Wen S.W., Acheampong K., Liu A. Omentin-1 in diabetes mellitus: A systematic review and meta-analysis. PLoS One 2019; 14(12): e0226292, doi: 10.1371/journal.pone.0226292.
50.
Eimal Latif A.H., Anwar S., Gautham K.S., Kadurei F., Ojo R.O., Hafizyar F. et al. Association of plasma omentin-1 levels with diabetes and its complications. Cureus 2021; 13(9): e18203, doi: 10.7759/cureus.18203.
51.
Biscetti F., Nardella E., Rando M.M., Cecchini A.L., Angelini F., Cina A. et al. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascularization in diabetic patients: a prospective cohort study. Cardiovasc. Diabetol. 2020: 19(1): 170, doi: 10.1186/s12933-020-01151-z.
52.
Askin L., Duman H., Ozyıldız A., Tanrıverdi O., Turkmen S. Association between omentin-1 and coronary artery disease: pathogenesis and clinical research. Curr. Cardiol. Rev. 2020; 16(3): 198–201, doi: 10.2174/1573403X16666200511085304.
CYTOWANIA (1):
1.
Exploring the role of adipocytokines in obesity and depression
Ruchi Keswani, Vaishnavi G. Thorat, C.R. Patil, Shvetank Bhatt
Drug Discovery Today
Ruchi Keswani, Vaishnavi G. Thorat, C.R. Patil, Shvetank Bhatt
Drug Discovery Today
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.