Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Potencjał terapeutyczny flawonoidów wykorzystywanych w tradycyjnej medycynie chińskiej – porównanie galanginy, kemferolu, chryzyny i fisetyny
1
Students’ Scientific Group, Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
2
Students’ Research Club, Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
Department of Community Pharmacy, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
4
Department of Organic Chemistry, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
Zaznaczeni autorzy mieli równy wkład w przygotowanie tego artykułu
Autor do korespondencji
Arkadiusz Sokal
Studenckie Koło Naukowe, Katedra i Zakład Chemii Organicznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jagiellońska 4, 41-200 Sosnowiec
Studenckie Koło Naukowe, Katedra i Zakład Chemii Organicznej, Wydział Nauk Farmaceutycznych w Sosnowcu, Śląski Uniwersytet Medyczny w Katowicach, ul. Jagiellońska 4, 41-200 Sosnowiec
Ann. Acad. Med. Siles. 2024;78:49-60
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
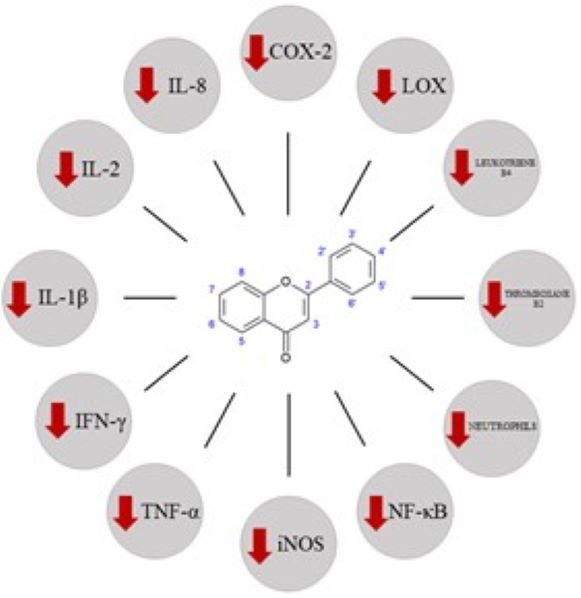
Ziołolecznictwo stanowi jedną z głównych gałęzi tradycyjnej medycyny chińskiej (traditional Chinese medicine – TCM). W pracy skupiono się na aktywności biologicznej wybranych flawonoidów, a także konkretnych przykładach wpływu tych substancji na różne układy organizmu. Flawonoidy to grupa związków chemicznych zawartych w surowcach roślinnych, miodzie, propolisie czy grzybach stosowanych w TCM. Chryzyna, galangina, kemferol i fisetyna to przykłady flawonoidów wykazujących m.in. właściwości przeciwutleniające, przeciwzapalne czy przeciwbakteryjne. Właściwości te są przedmiotem wielu badań naukowych, mających na celu zbadanie ich potencjalnego działania terapeutycznego.
REFERENCJE (116)
1.
Xiang Y., Guo Z., Zhu P., Chen J., Huang Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019; 8(5): 1958–1975, doi: 10.1002/cam4.2108.
2.
Marshall A.C. Traditional Chinese medicine and clinical pharmacology. Drug Discovery and Evaluation: Methods in Clinical Pharmacology 2020; 455–482, doi: 10.1007/978-3-319-68864-0_60.
3.
Wang X., Zhang A., Sun H., Yan G., Wang P., Han Y. Traditional Chinese medicine: current state, challenges, and applications. In: Serum Pharmacochemistry of Traditional Chinese Medicine: Technologies, Strategies and Applications. X. Wang [ed.]. Academic Press 2017, p. 1–6.
4.
Li Y.H., Chen F., Wang J.F., Wang Y., Zhang J.Q., Guo T. Analysis of nine compounds from Alpinia oxyphylla fruit at different harvest time using UFLC-MS/MS and an extraction method optimized by orthogonal design. Chem. Cent. J. 2013; 7(1): 134, doi: 134. 10.1186/1752-153X-7-134.
5.
Zheng M., Peng Z., Li J., Lin L., Peng S., Huang X. Optimization of extraction of galangin from galangal by response surface method. In: Proceedings of the 2016 6th International Conference on Machinery, Materials, Environment, Biotechnology and Computer. Atlantis Press 2016, p. 663–670, doi: 10.2991/mmebc-16.2016.141.
6.
Cid-Ortega S., Monroy-Rivera J.A. Extraction of kaempferol and its glycosides using supercritical fluids from plant sources: A review. Food Technol. Biotechnol. 2018; 56(4): 480–493, doi: 10.17113/ftb.56.04.18.5870.
7.
Surnis S.A., Patil P.S., Jadhav R.H. Extraction, isolation and quantification of bioactive compound (fisetin) and its product formulation. Int. J. Eng. Res. Technol. 2016; 5(8): 56–58, doi: 10.17577/IJERTV5IS080030.
8.
Dias M.C., Pinto D.C.G.A., Silva A.M.S. Plant flavonoids: chemical characteristics and biological activity. Molecules 2021; 26(17): 5377, doi: 10.3390/molecules26175377.
9.
He Y.Q., Zhou C.C., Yu L.Y., Wang L., Deng J.L., Tao Y.L. et al. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021; 163: 105224, doi: 10.1016/j.phrs.2020.105224.
10.
Li A., Chen L., Zhou W., Pan J., Gong D., Zhang G. Effects of baicalein and chrysin on the structure and functional properties of β-lactoglobulin. Foods 2022; 11(2): 165, doi: 10.3390/foods11020165.
11.
Hossain U., Das A.K., Ghosh S., Sil P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020; 145: 111738, doi: 10.1016/j.fct.2020.111738.
12.
Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016; 5: e47, doi: 10.1017/jns.2016.41.
13.
Ramesh P., Jagadeesan R., Sekaran S., Dhanasekaran A., Vimalraj S. Flavonoids: classification, function, and molecular mechanisms involved in bone remodelling. Front. Endocrinol. 2021; 12: 779638, doi: 10.3389/fendo.2021.779638.
14.
Shamsudin N.F., Ahmed Q.U., Mahmood S., Ali Shah S.A., Khatib A., Mukhtar S. et al. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022; 27(4): 1149, doi: 10.3390/molecules27041149.
15.
Rakha A., Umar N., Rabail R., Butt M.S., Kieliszek M., Hassoun A. et al. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022; 156: 113945, doi: 10.1016/j.biopha.2022.113945.
16.
Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients 2020; 12(2): 457, doi: 10.3390/nu12020457.
17.
Shamsudin N.F., Ahmed Q.U., Mahmood S., Shah S.A.A., Sarian M.N., Khattak M.M.A.K. et al. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci. 2022; 23(20): 12605, doi: 10.3390/ijms232012605.
18.
Al-Khayri J.M., Sahana G.R., Nagella P., Joseph B.V., Alessa F.M., Al-Mssallem M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022; 27(9): 2901, doi: 10.3390/molecules27092901.
19.
Elmegerhi S., Su C., Buglewicz D.J., Aizawa Y., Kato T.A. Effect of hydroxyl group position in flavonoids on inducing single‑stranded DNA damage mediated by cupric ions. Int. J. Mol. Med. 2018; 42(1): 658–664, doi: 10.3892/ijmm.2018.3615.
20.
Guerrero F.A., Medina G.M. Effect of a medicinal plant (Passiflora incarnata L) on sleep. Sleep Sci. 2017; 10(3): 96–100, doi: 10.5935/1984-0063.20170018.
21.
Mani R., Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018; 145: 187–196, doi: 10.1016/j.phytochem.2017.09.016.
22.
Sundaraganesan N., Mariappan G., Manoharan S. Molecular structure and vibrational spectroscopic studies of chrysin using HF and Density Functional Theory. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2012; 87: 67–76, doi: 10.1016/j.saa.2011.11.011.
23.
Talebi M., Talebi M., Farkhondeh T., Kopustinskiene D.M., Simal-Gandara J., Bernatoniene J. et al. An updated review on the versatile role of chrysin in neurological diseases: chemistry, pharmacology, and drug delivery approaches. Biomed. Pharmacother. 2021; 141: 111906, doi: 10.1016/j.biopha.2021.111906.
24.
Farkhondeh T., Samarghandian S., Bafandeh F. The cardiovascular protective effects of chrysin: A narrative review on experimental researches. Cardiovasc. Hematol. Agents Med. Chem. 2019; 17(1): 17–27, doi: 10.2174/1871525717666190114145137.
25.
He Y., Xia Z., Yu D., Wang J., Jin L., Huang D. et al. Hepatoprotective effects and structure-activity relationship of five flavonoids against lipopolysaccharide/d-galactosamine induced acute liver failure in mice. Int. Immunopharmacol. 2019; 68: 171–178, doi: 10.1016/j.intimp.2018.12.059.
26.
Angelopoulou E., Pyrgelis E.S., Piperi C. Neuroprotective potential of chrysin in Parkinson’s disease: Molecular mechanisms and clinical implications. Neurochem. Int. 2020; 132: 104612, doi: 10.1016/j.neuint.2019.104612.
27.
Wang M., Tao L., Xu H. Chinese herbal medicines as a source of molecules with anti-enterovirus 71 activity. Chin. Med. 2016; 11: 2, doi: 10.1186/s13020-016-0074-0.
28.
Bhat S.A., Hasan S.K., Parray Z.A., Siddiqui Z.I., Ansari S., Anwer A. et al. Potential antiviral activities of chrysin against hepatitis B virus. Gut Pathog. 2023; 15(1): 11, doi: 10.1186/s13099-023-00531-6.
29.
Souza L.C., Antunes M.S., Filho C.B., Del Fabbro L., de Gomes M.G., Goes A.T.R. et al. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015; 134: 22–30, doi: 10.1016/j.pbb.2015.04.010.
30.
He B., Xu F., Yan T., Xiao F., Wu B., Wang Y. et al. Tectochrysin from Alpinia Oxyphylla Miq. alleviates Aβ1–42 induced learning and memory impairments in mice. Eur. J. Pharmacol. 2019; 842: 365–372, doi: 10.1016/j.ejphar.2018.11.002.
31.
Qi Y., Cheng X., Jing H., Yan T., Xiao F., Wu B. et al. Effect of Alpinia oxyphylla–Schisandra chinensis herb pair on inflammation and apoptosis in Alzheimer’s disease mice model. J. Ethnopharmacol. 2019; 237: 28–38, doi: 10.1016/j.jep.2019.03.029.
32.
Giacomeli R., de Gomes M.G., Reolon J.B., Haas S.E., Colomé L.M., Jesse C.R. Chrysin loaded lipid-core nanocapsules ameliorates neurobehav-ioral alterations induced by β-amyloid1-42 in aged female mice. Behav. Brain Res. 2020; 390: 112696, doi: 10.1016/j.bbr.2020.112696.
33.
Sharma P., Kumari A., Gulati A., Krishnamurthy S., Hemalatha S. Chrysin isolated from Pyrus pashia fruit ameliorates convulsions in experimental animals. Nutr. Neurosci. 2019; 22(8): 569–577, doi: 10.1080/1028415X.2017.1418786.
34.
Shang J., Jiao J., Yan M., Wang J., Li Q., Shabuerjiang L. et al. Chrysin protects against cerebral ischemia-reperfusion injury in hippocampus via restraining oxidative stress and transition elements. Biomed. Pharmacother. 2023; 161: 114534, doi: 10.1016/j.biopha.2023.114534.
35.
Singh B., Singh D., Goel R.K. Dual protective effect of Passiflora incarnata in epilepsy and associated post-ictal depression. J. Ethnopharmacol. 2012; 139(1): 273–279, doi: 10.1016/j.jep.2011.11.011.
36.
Mishra A., Mishra P.S., Bandopadhyay R., Khurana N., Angelopoulou E., Paudel Y.N. et al. Neuroprotective potential of chrysin: mechanistic insights and therapeutic potential for neurological disorders. Molecules 2021; 26(21): 6456, doi: 10.3390/molecules26216456.
37.
Ghaderi S., Komaki A., Salehi I., Basir Z., Rashno M. Possible mechanisms involved in the protective effects of chrysin against lead-induced cognitive decline: an in vivo study in a rat model. Biomed. Pharmacother. 2023; 157: 114010, doi: 10.1016/j.biopha.2022.114010.
38.
Anandhi R., Annadurai T., Anitha T.S., Muralidharan A.R., Najmunnisha K., Nachiappan V. et al. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. J. Physiol. Biochem. 2013; 69(2): 313–323, doi: 10.1007/s13105-012-0215-6.
39.
Veerappan R., Malarvili T. Chrysin pretreatment improves angiotensin system, cGMP concentration in L-NAME induced hypertensive rats. Indian J. Clin. Biochem. 2019; 34(3): 288–295, doi: 10.1007/s12291-018-0761-y.
40.
Testai L., Martelli A., Cristofaro M., Breschi M.C., Calderone V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013; 65(5): 750–756, doi: 10.1111/jphp.12032.
41.
Zhao S., Liang M., Wang Y., Hu J., Zhong Y., Li J. et al. Chrysin suppresses vascular endothelial inflammation via inhibiting the NF-κB signaling pathway. J. Cardiovasc. Pharmacol. Ther. 2019; 24(3): 278–287, doi: 10.1177/1074248418810809.
42.
Ma G., Zhang J., Yang X., Guo P., Hou X., Fan Y. et al. TMEM16A-encoded anoctamin 1 inhibition contributes to chrysin-induced coronary relaxation. Biomed. Pharmacother. 2020; 131: 110766, doi: 10.1016/j.biopha.2020.110766.
43.
Anghel N., Cotoraci C., Ivan A., Suciu M., Herman H., Balta C. et al. Chrysin attenuates cardiomyocyte apoptosis and loss of intermediate filaments in a mouse model of mitoxantrone cardiotoxicity. Histol. Histopathol. 2015; 30(12): 1465–1475, doi: 10.14670/HH-11-641.
44.
Pérez-Lozano M.L., Cesaro A., Mazor M., Esteve E., Berteina-Raboin S., Best T.M. et al. Emerging natural-product-based treatments for the management of osteoarthritis. Antioxidants 2021; 10(2): 265, doi: 10.3390/antiox10020265.
45.
Oršolić, N., Nemrava J., Jeleč Ž., Kukolj M., Odeh D., Jakopović B. et al. Antioxidative and anti-inflammatory activities of chrysin and naringenin in a drug-induced bone loss model in rats. Int. J. Mol. Sci. 2022; 23(5): 2872, doi: 10.3390/ijms23052872.
46.
Lee J.J., Ng S.C., Hsu J.Y., Liu H., Chen C.J., Huang C.Y. et al. Galangin reverses H2O2-induced dermal fibroblast senescence via SIRT1-PGC-1α/Nrf2 signaling. Int. J. Mol. Sci. 2022; 23(3): 1387, doi: 10.3390/ijms23031387.
47.
Zhang X., Xie Z., Chen X., Qiu J., Tan Y., Li X. et al. Herb-drug interaction in the protective effect of Alpinia officinarum against gastric injury induced by indomethacin based on pharmacokinetic, tissue distribution and excretion studies in rats. J. Pharm. Anal. 2021; 11(2): 200–209, doi: 10.1016/j.jpha.2020.05.009.
48.
Chen Q.X., Zhou L., Long T., Qin D.L., Wang Y.L., Ye Y. et al. Galangin exhibits neuroprotective effects in 6-OHDA-induced models of Parkinson’s disease via the Nrf2/Keap1 pathway. Pharmaceuticals 2022; 15(8): 1014, doi: 10.3390/ph15081014.
49.
Liu C., Fan F., Zhong L., Su J., Zhang Y., Tu Y. Elucidating the material basis and potential mechanisms of Ershiwuwei Lvxue Pill acting on rheumatoid arthritis by UPLC-Q-TOF/MS and network pharmacology. PLoS One 2022; 17(2): e0262469, doi: 10.1371/journal.pone.0262469.
50.
Zhong X., Huang S., Liu D., Jiang Z., Jin Q., Li C. et al. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer Med. 2020; 9(15): 5546–5557, doi: 10.1002/cam4.3195.
51.
Atwa G., Omran G., Elbaky A.A., Okda T. The antitumour effect of galangin and luteolin with doxorubicin on chemically induced hepatocellular carcinoma in rats. Contemp. Oncol. 2021; 25(3): 174–184, doi: 10.5114/wo.2021.110048.
52.
Fang D., Xiong Z., Xu J., Yin J., Luo R. Chemopreventive mechanisms of galangin against hepatocellular carcinoma: A review. Biomed. Pharmacother. 2019; 109: 2054–2061, doi: 10.1016/j.biopha.2018.09.154.
53.
Xiong Y., Lai X., Xiang W., Zhou J., Han J., Li H. et al. Galangin (GLN) suppresses proliferation, migration, and invasion of human glioblastoma cells by targeting Skp2-induced epithelial-mesenchymal transition (EMT). OncoTargets Ther. 2020; 13: 9235–9244, doi: 10.2147/OTT.S264209.
54.
Rampogu S., Gajula R.G., Lee K.W. A comprehensive review on chemotherapeutic potential of galangin. Biomed. Pharmacother. 2021; 141: 111808, doi: 10.1016/j.biopha.2021.111808.
55.
Aladaileh S.H., Al-Swailmi F.K., Abukhalil M.H., Shalayel M.H. Galangin protects against oxidative damage and attenuates inflammation and apoptosis via modulation of NF-κB P65 and caspase-3 signaling molecules in a rat model of diabetic nephropathy. J. Physiol. Pharmacol. 2021; 72(1), doi: 10.26402/jpp.2021.1.04.
56.
Liao J., Liu B., Chen K., Hu S., Liu Z.Y., Li Y.X. et al. Galangin attenuates oxidative stress-mediated apoptosis in high glucose-induced renal tubular epithelial cells through modulating renin–angiotensin system and PI3K/AKT/mTOR pathway. Toxicol. Res. 2021; 10(3): 551–560, doi: 10.1093/toxres/tfab009.
57.
Kalhotra P., Chittepu V.C.S.R., Osorio-Revilla G., Gallardo-Velázquez T. Discovery of galangin as a potential DPP-4 inhibitor that improves insulin-stimulated skeletal muscle glucose uptake: A combinational therapy for diabetes. Int. J. Mol. Sci. 2019; 20(5): 1228, doi: 10.3390/ijms20051228.
58.
Abukhalil M.H., Althunibat O.Y., Aladaileh S.H., Al-Amarat W., Obeidat H.M., Al-Khawalde A.A.A. et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021; 138: 111410, doi: 10.1016/j.biopha.2021.111410.
59.
Aloud A.A., Chinnadurai V., Chandramohan G., Alsaif M.A., Al-Numair K.S. Galangin controls streptozotocin-caused glucose homeostasis and reverses glycolytic and gluconeogenic enzyme changes in rats. Arch. Physiol. Biochem. 2020; 126(2): 101–106, doi: 10.1080/13813455.2018.1498521.
60.
Jiang H., Li M., Du K., Ma C., Cheng Y., Wang S. et al. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin. Med. 2021; 16(1): 129, doi: 10.1186/s13020-021-00539-7.
61.
Liu Y.Y., Yu L.H., Zhang J., Xie D.J., Zhang X.X., Yu J.M. Network pharmacology-based and molecular docking-based analysis of Suanzaoren decoction for the treatment of Parkinson’s disease with sleep disorder. Biomed Res. Int. 2021; 2021: 1752570, doi: 10.1155/2021/1752570.
62.
Li T., Zhang L., Jin C., Xiong Y., Cheng Y.Y., Chen K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020; 131: 110769, doi: 10.1016/j.biopha.2020.110769.
63.
Dabeek W.M., Marra M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019; 11(10): 2288, doi: 10.3390/nu11102288.
64.
Ren J., Lu Y., Qian Y., Chen B., Wu T., Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019; 18(4): 2759–2776, doi: 10.3892/etm.2019.7886.
65.
Cui Y., Wang H., Wang D., Mi J., Chen G., Li F. et al. Network pharmacology analysis on the mechanism of Huangqi Sijunzi Decoction in treating cancer-related fatigue. J. Healthc. Eng. 2021; 2021: 9780677, doi: 10.1155/2021/9780677.
66.
Lyu M., Wang Y., Chen Q., Qin J., Hou D., Huang S. et al. Molecular mechanism underlying effects of Wumeiwan on steroid-dependent asthma: A network pharmacology, molecular docking, and experimental verification study. Drug Des. Devel. Ther. 2022; 16: 909–929, doi: 10.2147/DDDT.S349950.
67.
Wei M., Li H., Li Q., Qiao Y., Ma Q., Xie R. et al. Based on network pharmacology to explore the molecular targets and mechanisms of Gegen Qinlian decoction for the treatment of ulcerative colitis. Biomed Res. Int. 2020; 2020: 5217405, doi: 10.1155/2020/5217405.
68.
Yin B., Bi Y.M., Fan G.J., Xia Y.Q. Molecular mechanism of the effect of Huanglian Jiedu Decoction on type 2 diabetes mellitus based on network pharmacology and molecular docking. J. Diabetes Res. 2020; 2020: 5273914, doi: 10.1155/2020/5273914.
69.
He D., Huang J.H., Zhang Z.Y., Du Q., Peng W.J., Yu R. et al. A network pharmacology-based strategy for predicting active ingredients and potential targets of LiuWei DiHuang Pill in treating type 2 diabetes mellitus. Drug Des. Devel. Ther. 2019; 13: 3989–4005, doi: 10.2147/DDDT.S216644.
70.
Shi H., Tian S., Tian H. Network pharmacology interpretation of Fuzheng-Jiedu Decoction against colorectal cancer. Evid. Based Complement. Alternat. Med. 2021; 2021: 4652492, doi: 10.1155/2021/4652492.
71.
He J., Wo D., Ma E., Wang Q., Chen J., Peng J. et al. Network pharmacology-based analysis in determining the mechanisms of Huoxin pill in protecting against myocardial infarction. Pharm. Biol. 2021; 59(1): 1191–1202, doi: 10.1080/13880209.2021.1964542.
72.
Tang M., Xie X., Yi P., Kang J., Liao J., Li W. et al. Integrating network pharmacology with molecular docking to unravel the active compounds and potential mechanism of simiao pill treating rheumatoid arthritis. Evid. Based Complement. Alternat. Med. 2020; 2020: 5786053, doi: 10.1155/2020/5786053.
73.
Hu Q.H., Jiao R.Q., Wang X., Lv Y.Z., Kong L.D. Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J. Ethnopharmacol. 2010; 128(3): 685–692, doi: 10.1016/j.jep.2010.02.012.
74.
Huang J., Zhao L., Sun J., Wang L., Gu J., Liu X. et al. Clinical evidence and potential mechanisms of complementary treatment of Ling Gui Zhu Gan formula for the management of serum lipids and obesity. Evid. Based Complement. Alternat. Med. 2022; 2022: 7714034, doi: 10.1155/2022/7714034.
75.
Chang S., Li X., Zheng Y., Shi H., Zhang D., Jing B. et al. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF‐ĸB signaling pathway. Phytother. Res. 2022; 36(4): 1678–1691, doi: 10.1002/ptr.7396.
76.
Silva dos Santos J., Gonçalves Cirino J.P., de Oliveira Carvalho P., Ortega M.M. The pharmacological action of kaempferol in central nervous system diseases: A review. Front. Pharmacol. 2021; 11: 565700, doi: 10.3389/fphar.2020.565700.
77.
Wang J., Mao J., Wang R., Li S. ,Wu B., Yuan Y. Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Front. Pharmacol. 2020; 11: 424, doi: 10.3389/fphar.2020.00424.
78.
Jantas D., Malarz J., Le T.N., Stojakowska A. Neuroprotective properties of kempferol derivatives from Maesa membranacea against oxidative stress-induced cell damage: An association with cathepsin D inhibition and PI3K/Akt activation. Int. J. Mol. Sci. 2021; 22(19): 10363, doi: 10.3390/ijms221910363.
79.
Abdelsalam S.A., Renu K., Zahra H.A., Abdallah B.M., Ali E.M., Veeraraghavan V.P., Sivalingam K. et al. Polyphenols mediate neuroprotection in cerebral ischemic stroke–An update. Nutrients 2023; 15(5): 1107, doi: 10.3390/nu15051107.
80.
Lee S., Seol H.S., Eom S., Lee J., Kim C., Park J.H. et al. Hydroxy pentacyclic triterpene acid, kaempferol, inhibits the human 5-Hydro-xytryptamine type 3A receptor activity. Int. J. Mol. Sci. 2022; 23(1): 544, doi: 10.3390/ijms23010544.
81.
Moratilla-Rivera I., Sánchez M., Valdés-González J.A., Gómez-Serranillos M.P. Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int. J. Mol. Sci. 2023; 24(4): 3748, doi: 10.3390/ijms24043748.
82.
Zhang S.S., Liu M., Liu D.N., Shang Y.F., Du G.H., Wang Y.H. Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. Int. J. Mol. Sci. 2022; 23(20): 12694, doi: 10.3390/ijms232012694.
83.
Inden M., Takagi A., Kitai H., Ito T., Kurita H., Honda R. et al. Kaempferol has potent protective and antifibrillogenic effects for α-synuclein neurotoxicity in vitro. Int. J. Mol. Sci. 2021; 22(21): 11484, doi: 10.3390/ijms222111484.
84.
Koga S., Sekiya H., Kondru N., Ross O.A., Dickson D.W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 2021; 16(1): 83, doi: 10.1186/s13024-021-00501-z.
85.
Periferakis, A., Periferakis K., Badarau I.A., Petran E.M., Popa D.C., Caruntu A. et al. Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022; 23(23): 15054, doi: 10.3390/ijms232315054.
86.
Naim N., Bouymajane A., Oulad El Majdoub Y., Ezrari S., Lahlali R., Tahiri A. et al. Flavonoid composition and antibacterial properties of Crocus sativus L. petal extracts. Molecules 2022; 28(1): 186, doi: 10.3390/molecules28010186.
87.
Zhou H., Xu M., Guo W., Yao Z., Du X., Chen L. et al. The antibacterial activity of kaempferol combined with colistin against colistin-resistant Gram-negative bacteria. Microbiol. Spectr. 2022; 10(6): e0226522, doi: 10.1128/spectrum.02265-22.
88.
He X., Zhang W., Cao Q., Li Y., Bao G., Lin T. et al. Global downregulation of penicillin resistance and biofilm formation by MRSA is associated with the interaction between kaempferol rhamnosides and quercetin. Microbiol. Spectr. 2022; 10(6): e0278222, doi: 10.1128/spectrum.02782-22.
89.
Venmathi Maran B.A., Iqbal M., Gangadaran P., Ahn B.C., Rao P.V., Shah M.D. Hepatoprotective potential of Malaysian medicinal plants: A review on phytochemicals, oxidative stress, and antioxidant mechanisms. Molecules 2022; 27(5): 1533, doi: 10.3390/molecules27051533.
90.
Alkandahri M.Y., Pamungkas B.T., Oktoba Z., Shafirany M.Z., Sulastri L., Arfania M. et al. Hepatoprotective effect of kaempferol: A review of the dietary sources, bioavailability, mechanisms of action, and safety. Adv. Pharmacol. Pharm. Sci. 2023; 2023: 1387665, doi: 10.1155/2023/1387665.
91.
Asaad G.F., Ibrahim Abdallah H.M., Mohammed H.S., Nomier Y.A. Hepatoprotective effect of kaempferol glycosides isolated from Cedrela odorata L. leaves in albino mice: involvement of Raf/MAPK pathway. Res. Pharm. Sci. 2021; 16(4): 370–380, doi: 10.4103/1735-5362.319575.
92.
BinMowyna M.N., AlFaris N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021; 59(1): 146–156, doi: 10.1080/13880209.2021.1877734.
93.
Kashyap D., Sharma A., Tuli H. S., Sak K., Punia S., Mukherjee T.K. Kaempferol – a dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017; 30: 203–219, doi: 10.1016/j.jff.2017.01.022.
94.
Rajendran P., Ammar R.B., Al-Saeedi F.J., Mohamed M.E., ElNaggar M.A. Al-Ramadan S.Y. et al. Kaempferol inhibits zearalenone-induced oxidative stress and apoptosis via the PI3K/Akt-mediated Nrf2 signaling pathway: in vitro and in vivo studies. Int. J. Mol. Sci. 2020; 22(1): 217, doi: 10.3390/ijms22010217.
95.
Chen Y., Li T., Tan P., Shi H., Cheng Y., Cai T. et al. Kaempferol from Penthorum chinense Pursh attenuates hepatic ischemia/reperfusion injury by suppressing oxidative stress and inflammation through activation of the Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2022; 13: 857015, doi: 10.3389/fphar.2022.857015.
96.
Lee C., Yoon S., Moon J.O. Kaempferol suppresses carbon tetrachloride-induced liver damage in rats via the MAPKs/NF-κB and AMPK/Nrf2 signaling pathways. Int. J. Mol. Sci. 2023; 24(8): 6900, doi: 10.3390/ijms24086900.
97.
Rosado-Ramos R., Godinho-Pereira J., Marques D., Figueira I., Fleming Outeiro T., Menezes R. et al. Small molecule fisetin modulates alpha–synuclein aggregation. Molecules 2021; 26(11): 3353, doi: 10.3390/molecules26113353.
98.
Zhi G., Shao B., Zheng T., Mu J., Li J., Feng Y. et al. Exploring the molecular mechanism of Gan Shuang granules for the treatment of non-alcoholic steatohepatitis using network pharmacology, molecular docking, and experimental verification. Front. Pharmacol. 2023; 14: 1082451, doi: 10.3389/fphar.2023.1082451.
99.
Huang Z., Guo S., Fu C., Zhou W., Stalin A., Zhang J. et al. Identification of molecular mechanisms underlying the therapeutic effects of Xintong granule in coronary artery disease by a network pharmacology and molecular docking approach. Medicine 2022; 101(27): e29829, doi: 10.1097/MD.0000000000029829.
100.
Wu Z., Yang Q., Ma H. Study the mechanism of Gualou Niubang decoction in treating plasma cell mastitis based on network pharmacology and molecular docking. Biomed Res. Int. 2022; 2022: 5780936, doi: 10.1155/2022/5780936.
101.
Feng T., Zhang M., Xu Q., Song F., Wang L., Gai S. et al. Exploration of molecular targets and mechanisms of Chinese medicinal formula Acacia Catechu–Scutellariae Radix in the treatment of COVID‐19 by a systems pharmacology strategy. Phytother. Res. 2022; 36(11): 4210–4229, doi: 10.1002/ptr.7554.
102.
Kubina R., Krzykawski K., Kabała-Dzik A., Wojtyczka R.D., Chodurek E., Dziedzic A. Fisetin, a potent anticancer flavonol exhibiting cytotoxic activity against neoplastic malignant cells and cancerous conditions: A scoping, comprehensive review. Nutrients 2022; 14(13): 2604, doi: 10.3390/nu14132604.
103.
Imran M., Saeed F., Gilani S.A., Shariati M.A., Imran A., Afzaal M. et al. Fisetin: an anticancer perspective. Food Sci. Nutr. 2020; 9(1): 3–16, doi: 10.1002/fsn3.1872.
104.
Ding H., Li Y., Chen S., Wen Y., Zhang S., Luo E. et al. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis‐associated encephalopathy. CNS Neurosci. Ther. 2022; 28(2): 247–258, doi: 10.1111/cns.13765.
105.
Wu S.J., Huang W.C., Cheng C.Y., Wang M.C., Cheng S.C., Liou C.J. Fisetin suppresses the inflammatory response and oxidative stress in bronchial epithelial cells. Nutrients 2022; 14(9): 1841, doi: 10.3390/nu14091841.
106.
Sok Yen F., Shu Qin C., Tan Shi Xuan S., Jia Ying P., Yi Le H., Darmarajan T. et al. Hypoglycemic effects of plant flavonoids: A review. Evid. Based Complement. Alternat. Med. 2021; 2021: 2057333, doi: 10.1155/2021/2057333.
107.
Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020; 288(5): 518–536, doi: 10.1111/joim.13141.
108.
Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018; 36: 18–28, doi: 10.1016/j.ebiom.2018.09.015.
109.
Wissler Gerdes E.O., Zhu Y., Weigand B.M., Tripathi U., Burns T.C., Tchkonia T. et al. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int. Rev. Neurobiol. 2020; 155: 203–234, doi: 10.1016/bs.irn.2020.03.019.
110.
Verdoorn B.P., Evans T.K., Hanson G.J., Zhu Y., Langhi Prata L.G.P., Pignolo R.J. et al. Fisetin for COVID‐19 in skilled nursing facilities: senolytic trials in the COVID era. J. Am. Geriatr. Soc. 2021; 69(11): 3023–3033, doi: 10.1111/jgs.17416.
111.
Hambright W.S., Mu X., Gao X., Guo P., Kawakami Y., Mitchell J. et al. The senolytic drug fisetin attenuates bone degeneration in the Zmpste24−/− progeria mouse model. J. Osteoporos. 2023; 2023: 5572754, doi: 10.1155/2023/5572754.
112.
Deledda A., Giordano E., Velluzzi F., Flore G., Franceschelli S., Speranza L. et al. Mitochondrial aging and senolytic natural products with protective potential. Int. J. Mol. Sci. 2022; 23(24): 16219, doi: 10.3390/ijms232416219.
113.
Russo M., Moccia S., Luongo D., Russo G.L. Senolytic flavonoids enhance type-I and type-II cell death in human radioresistant colon cancer cells through AMPK/MAPK pathway. Cancers 2023; 15(9): 2660, doi: 10.3390/cancers15092660.
114.
Hassan S.S.U., Samanta S., Dash R., Karpiński T.M., Habibi E., Sadiq A. et al. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022; 13: 1015835, doi: 10.3389/fphar.2022.1015835.
115.
Elsallabi O., Patruno A., Pesce M., Cataldi A., Carradori S., Gallorini M. Fisetin as a senotherapeutic agent: Biopharmaceutical properties and cross-talk between cell senescence and neuroprotection. Molecules 2022; 27(3): 738, doi: 10.3390/molecules27030738.
116.
Maher P. Preventing and treating neurological disorders with the flavonol fisetin. Brain Plast. 2021; 6(2): 155–166, doi: 10.3233/BPL-200104.
CYTOWANIA (2):
1.
Potential Strategies for Overcoming Drug Resistance Pathways Using Propolis and Its Polyphenolic/Flavonoid Compounds in Combination with Chemotherapy and Radiotherapy
Nada Oršolić, Maja Jazvinšćak Jembrek
Nutrients
Nada Oršolić, Maja Jazvinšćak Jembrek
Nutrients
2.
In silico and in vivo evaluations of fisetin and fisetin-loaded nanosuspension on monoamine oxidase inhibition in Aβ(25-35) induced dementia in mice model
Siti Zaidathul Iman Zolkiffly, Mizaton Hazizul Hasan, Siti Azma Jusoh, Ashok Kumar Janakiraman, Sathesh Kumar Sukumaran, Noreen Husain, Yuslina Zakaria, Hanish Singh Jayasingh Chellammal
Pharmacological Research - Modern Chinese Medicine
Siti Zaidathul Iman Zolkiffly, Mizaton Hazizul Hasan, Siti Azma Jusoh, Ashok Kumar Janakiraman, Sathesh Kumar Sukumaran, Noreen Husain, Yuslina Zakaria, Hanish Singh Jayasingh Chellammal
Pharmacological Research - Modern Chinese Medicine
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.