Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Wpływ anestetyków na transkryptom
1
Students’ Scientific Club at the Department of Forensic Medicine and Toxicology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
2
Department of Forensic Medicine and Toxicology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Marcin Tomsia
Katedra i Zakład Medycyny Sądowej i Toksykologii Sądowo-Lekarskiej, ul. Medyków 18, 40-752 Katowice
Katedra i Zakład Medycyny Sądowej i Toksykologii Sądowo-Lekarskiej, ul. Medyków 18, 40-752 Katowice
Ann. Acad. Med. Siles. 2025;79:119-131
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Środki znieczulające są rutynowo stosowane jako kluczowy element wielu zabiegów, niewiele jednak wiadomo o długotrwałych skutkach ich stosowania. W zależności od mechanizmu działania anestetyki różnią się pod względem skuteczności i częstości występowania działań niepożądanych. Celem badania jest przegląd najnowszej literatury na temat wpływu środków znieczulających na transkryptom i organizm człowieka. Przedstawiono szeroki zakres zmian powstających w wyniku stosowania anestetyków, a także ich działanie mogące wykraczać poza okres okołooperacyjny. Przybliżono pewne funkcje anestetyków w procesie nowotworowym, takie jak zwiększenie i zmniejszenie ekspresji genów czy osłabienie elementów układu odpornościowego. Podkreślono obiecującą rolę środków miejscowo znieczulających w ograniczaniu nawrotów nowotworu. Opisano również problem narażenia dużych grup specjalistów pracujących na salach operacyjnych i oddziałach klinicznych. Badania potwierdziły, że anestetyki nie są obojętne dla ludzkiego organizmu; wpływają na układ immunologiczny, mogą wywierać działanie rakotwórcze, wpływać na proliferację, różnicowanie komórek, a także odgrywać rolę we wzroście guza i rozwoju przerzutów nowotworowych. Konieczne są nowe badania nad wpływem leków znieczulających. Wyniki tych badań mogą zapewnić nowy cel terapeutyczny, a odpowiednio dobrana terapia może zwiększyć szansę dobrego rokowania klinicznego, zmniejszyć prawdopodobieństwo wystąpienia zaburzeń behawioralnych i neurokognitywnych oraz ograniczyć rozprzestrzenianie się przerzutów.
REFERENCJE (89)
1.
Meara J.G., Leather A.J., Hagander L., Alkire B.C., Alonso N., Ameh E.A. et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Int. J. Obstet. Anesth. 2016; 25: 75–78, doi: 10.1016/j.ijoa.2015.09.006.
2.
Liu Y., Ding M., Gao Q., He A., Liu Y., Mei H. Current advances on the important roles of enhancer RNAs in gene regulation and cancer. Biomed. Res. Int. 2018; 2018: 2405351, doi: 10.1155/2018/2405351.
3.
Sakamoto A., Imai J.I., Nishikawa A., Honma R., Ito E., Yanagisawa Y. et al. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene 2005; 356: 39–48, doi: 10.1016/j.gene.2005.03.022.
4.
Kvolik S., Dobrosevic B., Marczi S., Prlic L., Glavas-Obrovac L. Different apoptosis ratios and gene expressions in two human cell lines after sevoflurane anaesthesia. Acta Anaesthesiol. Scand. 2009; 53(9): 1192–1199, doi: 10.1111/j.1399-6576.2009.02036.x.
5.
Benzonana L.L., Perry N.J.S., Watts H.R., Yang B., Perry I.A., Coombes C. et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013; 119(3): 593–605, doi: 10.1097/ALN.0b013e31829e47fd.
6.
Clar D.T., Patel S., Richards J.R. Anesthetic Gases. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
7.
Szrama J., Kusza K., Sobczyński P., Molnar Z., Siemionow M. The effect of volatile anesthetics on cellular responses in the microcirculation of free tissue transfers. Postepy Kardiol. Interwencyjnej 2022; 18(4): 459–464, doi: 10.5114/aic.2021.110926.
8.
Bara M., Janczak A. Toxicity of anesthetic gases: exposure in operating rooms and influence on the environment. Prosp. Pharm. Sci. 2023; 21(3): 1–5, doi: 10.56782/pps.157.
9.
Rozov S., Saarreharju R., Khirug S., Storvik M., Rivera C., Rantamäki T. Effects of nitrous oxide and ketamine on electrophysiological and molecular responses in the prefrontal cortex of mice: A comparative study. Eur. J. Pharmacol. 2024; 968: 176426, doi: 10.1016/j.ejphar.2024.176426.
10.
Gernez E., Lee G.R., Niguet J.P., Zerimech F., Bennis A., Grzych G. Nitrous oxide abuse: Clinical outcomes, pharmacology, pharmacokinetics, toxicity and impact on metabolism. Toxics 2023; 11(12): 962, doi: 10.3390/toxics11120962.
11.
Luan T., Li Y., Sun L., Xu S., Wang H., Wang J. et al. Systemic immune effects of anesthetics and their intracellular targets in tumors. Front. Med. (Lausanne) 2022; 9: 810189, doi: 10.3389/fmed.2022.810189.
12.
Agani F., Jiang B.H. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/ AKT/mTOR pathway in cancer. Curr. Cancer Drug Targets 2013; 13(3): 245–251, doi: 10.2174/1568009611313030003.
13.
Hoggard A., Shienbaum R., Mokhtar M., Singh P. Gaseous Anesthetics. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
15.
Kaymak C., Kadioglu E., Coskun E., Basar H., Basar M. Determination of DNA damage after exposure to inhalation anesthetics in human peripheral lymphocytes and sperm cells in vitro by comet assay. Hum. Exp. Toxicol. 2012; 31(12): 1207–1213, doi: 10.1177/0960327112446818.
16.
Kupczewska-Dobecka M., Dobecki M. Enflurane. Documentation of proposed values of occupational exposure limits (OELs). [Article in Polish]. Podstawy Metody Oceny Śr. Pr. 2023; 1(115): 45–89, doi: 10.54215/PiMOSP/3.115.2023.
17.
Deiner S., Silverstein J.H. Postoperative delirium and cognitive dysfunction. Br. J. Anaesth. 2009; 103(Suppl 1): i41–46, doi: 10.1093/bja/aep291.
18.
Zhu Z., Ma L. Sevoflurane induces inflammation in primary hippocampal neurons by regulating Hoxa5/Gm5106/miR-27b-3p positive feedback loop. Bioengineered 2021; 12(2): 12215–12226, doi: 10.1080/21655979.2021.2005927.
19.
Zhu X., Peng C., Peng Z., Chang R., Guo Q. Sevoflurane inhibits metastasis in hepatocellular carcinoma by inhibiting MiR-665-induced activation of the ERK/MMP pathway. Cell Transplant. 2022; 31: 9636897221104447, doi: 10.1177/09636897221104447.
20.
Jakobsson J. Desflurane: a clinical update of a third‐generation inhaled anaesthetic. Acta Anaesthesiol. Scand. 2012; 56(4): 420–432, doi: 10.1111/j.1399-6576.2011.02600.x.
21.
Akin A., Ugur F., Ozkul Y., Esmaoglu A., Gunes I., Ergul H. Desflurane anaesthesia increases sister chromatid exchanges in human lymphocytes. Acta Anaesthesiol. Scand. 2005; 49(10): 1559–1561, doi: 10.1111/j.1399-6576.2005.00779.x.
22.
Arruda N.M., Braz L.G., Nogueira F.R., Souza K.M., Aun A.G., Figueiredo D.B.S. et al. Inflammation and DNA damage induction in surgical patients maintained with desflurane anesthesia. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2019; 846: 403073, doi: 10.1016/j.mrgentox.2019.07.003.
23.
Melamed R., Bar-Yosef S., Shakhar G., Shakhar K., Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth. Analg. 2003; 97(5): 1331–1339, doi: 10.1213/01.ANE.0000082995.44040.07.
24.
Schneemilch C.E., Hachenberg T., Ansorge S., Ittenson A., Bank U. Effects of different anaesthetic agents on immune cell function in vitro. Eur. J. Anaesthesiol. 2005; 22(8): 616–623, doi: 10.1017/s0265021505001031.
25.
Snyder G.L., Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010; 105(2): 106–115, doi: 10.1093/bja/aeq164.
26.
Welters I.D., Hafer G., Menzebach A., Mühling J., Neuhäuser C., Browning P. et al. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth. Analg. 2010; 110(3): 934–941, doi: 10.1213/ANE.0b013e3181c95cfa.
27.
Zheng X., Wang Y., Dong L., Zhao S., Wang L., Chen H. et al. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospec-tive study. Onco Targets Ther. 2018; 11: 1141–1148, doi: 10.2147/OTT.S156792.
28.
Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018; 16(1): 8, doi: 10.1186/s12967-018-1389-7.
29.
Ponferrada A.R., Orriach J.L.G., Manso A.M., Haro E.S., Molina S.R., Heredia A.F. et al. Anaesthesia and cancer: can anaesthetic drugs modify gene expression? Ecancermedicalscience 2020; 14: 1080, doi: 10.3332/ecancer.2020.1080.
30.
Chen J.T., Wei L., Chen T.L., Huang C.J., Chen R.M. Regulation of cytochrome P450 gene expression by ketamine: a review. Expert Opin. Drug Metab. Toxicol. 2018; 14(7): 709–720, doi: 10.1080/17425255.2018.1487397.
31.
Hanych A., Borys M., Czuwar M. Ketamine in the treatment of neuropathic pain. [Article in Polish]. Anestezjologia i Ratownictwo 2020; 14(2): 201–208.
32.
Brogi E., Forfori F. Anesthesia and cancer recurrence: an overview. J. Anesth. Analg. Crit. Care 2022; 2(1): 33, doi: 10.1186/s44158-022-00060-9.
33.
Chang Y., Chen T.L., Sheu J.R., Chen R.M. Suppressive effects of ketamine on macrophage functions. Toxicol. Appl. Pharmacol. 2005; 204(1): 27–35, doi: 10.1016/j.taap.2004.08.011.
34.
Jafarzadeh A., Hadavi M., Hassanshahi G., Rezaeian M., Vazirinejad R. General anesthetics on immune system cytokines: a narrative review article. Anesth. Pain Med. 2020; 10(4): e103033, doi: 10.5812/aapm.103033.
35.
Jin Z., Mendu S.K., Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids 2013; 45(1): 87–94, doi: 10.1007/s00726-011-1193-7.
36.
Skibiski J., Patel P., Abdijadid S. Barbiturates. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
37.
Marik P.E. Propofol: an immunomodulating agent. Pharmacotherapy 2005; 25(5 Pt 2): 28S–33S, doi: 10.1592/phco.2005.25.5_part_2.28s.
38.
Miller R.D. Miller’s anesthesia. 7th ed. Churchill Livingstone. Philadelphia; 2010.
39.
Yi S., Tao X., Wang Y., Cao Q., Zhou Z., Wang S. Effects of propofol on macrophage activation and function in diseases. Front. Pharmacol. 2022; 13: 964771, doi: 10.3389/fphar.2022.964771.
40.
Lin Z., Bu H., Huang X. Examination of ADRB2 gene expression and influence of dexmedetomidine and propofol on hemodynamics after abdo-minal surgery. Cell. Mol. Biol. (Noisy-le-grand) 2023; 69(1): 87–92, doi: 10.14715/cmb/2022.69.1.15.
41.
Zhao J., Mo H. The impact of different anesthesia methods on stress reaction and immune function of the patients with gastric cancer during peri-operative period. J. Med. Assoc. Thai. 2015; 98(6): 568–573.
42.
Ben-Hamouda N., Poirel V.J., Dispersyn G., Pévet P., Challet E., Pain L. Short-term propofol anaesthesia down-regulates clock genes expression in the master clock. Chronobiol. Int. 2018; 35(12): 1735–1741, doi: 10.1080/07420528.2018.1499107.
43.
Eisenstein T.K. The role of opioid receptors in immune system func-tion. Front. Immunol. 2019; 10: 2904, doi: 10.3389/fimmu.2019.02904.
44.
Shavit Y., Wolf G., Goshen I., Livshits D., Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain 2005; 115(1–2): 50–59, doi: 10.1016/j.pain.2005.02.003.
45.
Byrne L.S., Peng J., Sarkar S., Chang S.L. Interleukin-1 beta-induced up-regulation of opioid receptors in the untreated and morphine-desensitized U87 MG human astrocytoma cells. J. Neuroinflammation 2012; 9:252, doi: 10.1186/1742-2094-9-252.
46.
Dai X., Song H.J., Cui S.G., Wang T,. Liu Q., Wang R. The stimulative effects of endogenous opioids on endothelial cell proliferation, migration and angiogenesis in vitro. Eur. J. Pharmacol. 2010; 628(1–3): 42–50, doi: 10.1016/j.ejphar.2009.11.035.
47.
Bai X., Huang Y., Zhang K., Huang W., Mu Y., Li Y. et al. CircNf1-mediated CXCL12 expression in the spinal cord contributes to morphine analgesic tolerance. Brain Behav. Immun. 2023; 107: 140–151, doi: 10.1016/j.bbi.2022.09.018.
48.
Deng M., Zhang Z., Xing M., Liang X., Li Z., Wu J. et al. LncRNA MRAK159688 facilitates morphine tolerance by promoting REST-mediated inhibition of mu opioid receptor in rats. Neuropharmacology 2022; 206: 108938, doi: 10.1016/j.neuropharm.2021.108938.
50.
Forget P., Collet V., Lavandʼhomme P., De Kock M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur. J. Anaesthesiol. 2010; 27(3): 233–240, doi: 10.1097/EJA.0b013e32832d540e.
51.
Beilin B., Shavit Y., Hart J., Mordashov B., Cohn S., Notti I. et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth. Analg. 1996; 82(3): 492–497, doi: 10.1097/00000539-199603000-00011.
52.
Sheng Z., Liu Q., Cheng C., Li M., Barash J., Kofke W.A. et al. Fentanyl induces autism-like behaviours in mice by hypermethylation of the glutamate receptor gene Grin2b. Br. J. Anaesth. 2022; 129(4): 544–554, doi: 10.1016/j.bja.2022.04.027.
53.
Grzanka A., Wasilewska I., Śliwczyńska M., Misiołek H. Hypersensitivity lo local anesthetics. Anaesthesiol. Intensive Ther. 2016; 48(2): 128–134, doi: 10.5603/AIT.a2016.0017.
54.
D’Agostino G., Saporito A., Cecchinato V., Silvestri Y., Borgeat A., Anselmi L. et al. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br. J. Anaesth. 2018; 121(4): 962–968, doi: 10.1016/j.bja.2018.07.015.
55.
Terkawi A.S., Durieux M.E., Gottschalk A., Brenin D., Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: a double-blind, placebo-controlled randomized trial. Reg. Anesth. Pain Med. 2014; 39(6): 472–477, doi: 10.1097/AAP.0000000000000140.
56.
Aird J., Baird A.M., Lim M.C.J., McDermott R., Finn S.P., Gray S.G. Carcinogenesis in prostate cancer: the role of long non-coding RNAs. Noncoding RNA Res. 2018; 3(1): 29–38, doi: 10.1016/j.ncrna.2018.01.001.
57.
Yamamoto H., Uchida Y., Chiba T., Kurimoto R., Matsushima T., Inotsume M. et al. Transcriptome analysis of sevoflurane exposure effects at the different brain regions. PLoS One 2020; 15(12): e0236771, doi: 10.1371/journal.pone.0236771.
58.
Chastain-Potts S.E., Tesic V., Tat Q.L., Cabrera O.H., Quillinan N., Jevtovic-Todorovic V. Sevoflurane exposure results in sex-specific transgenerational upregulation of target IEGs in the subiculum. Mol. Neurobiol. 2020; 57(1): 11–22, doi: 10.1007/s12035-019-01752-0.
59.
Oppenheim R.W. Cell death during development of the nervous system. Annu. Rev. Neurosci. 1991; 14(1): 453–501, doi: 10.1146/annurev.ne.14.030191.002321.
60.
Raff M.C., Barres B.A., Burne J.F., Coles H.S., Ishizaki Y., Jacobson M.D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science 1993; 262(5134): 695–700, doi: 10.1126/science.8235590.
61.
Rabinowicz T., de Courten-Myers G.M., Petetot J.M., Xi G., de los Reyes E. Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J. Neuropathol. Exp. Neurol. 1996; 55(3): 320–328.
62.
Campagna J.A., Miller K.W., Forman S.A. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 2003; 348(21): 2110–2124, doi: 10.1056/NEJMra021261.
63.
de Lima A.D., Opitz T., Voigt T. Irreversible loss of a subpopulation of cortical interneurons in the absence of glutamatergic network activity. Eur. J. Neurosci. 2004; 19(11): 2931–2943, doi: 10.1111/j.0953-816X.2004.03403.x.
64.
Coghlan M., Richards E., Shaik S., Rossi P., Vanama R.B., Ahmadi S. et al. Inhalational anesthetics induce neuronal protein aggregation and affect ER trafficking. Sci. Rep. 2018; 8(1): 5275, doi: 10.1038/s41598-018-23335-0.
65.
Tavare A.N., Perry N.J.S., Benzonana L.L., Takata M., Ma D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2012; 130(6): 1237–1250, doi: 10.1002/ijc.26448.
66.
Markovic S.N., Murasko D.M. Anesthesia inhibits interferon-induced natural killer cell cytotoxicity via induction of CD8+ suppressor cells. Cell. Immunol. 1993; 151(2): 474–480, doi: 10.1006/cimm.1993.1256.
67.
Gupta K., Kshirsagar S., Chang L., Schwartz R., Law P.Y., Yee D. et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002; 62(15): 4491–4498.
68.
Page G.G., McDonald J.S., Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br. J. Anaesth. 1998; 81(2): 216–223, doi: 10.1093/bja/81.2.216.
69.
Tegeder I., Grösch S., Schmidtko A., Häussler A., Schmidt H., Niederberger E. et al. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003; 63(8): 1846–1852.
70.
Oh T.K., Kim K., Jheon S., Lee J., Do S.H., Hwang J.W. et al. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control 2018; 25(1): 1073274818775360, doi: 10.1177/1073274818775360.
71.
Raytis J.L., Lew M.W. Surgical stress response and cancer metastasis: the potential benefit of perioperative beta blockade. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/b....
72.
Enlund M., Berglund A., Andreasson K., Cicek C., Enlund A., Bergkvist L. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups. J. Med. Sci. 2014; 119(3): 251–261, doi: 10.3109/03009734.2014.922649.
73.
Mokini Z., Cama A., Forget P. Anesthetics and long term cancer outcomes: may epigenetics be the key for pancreatic cancer? Medicina (Kaunas) 2022; 58(8): 1102, doi: 10.3390/medicina58081102.
74.
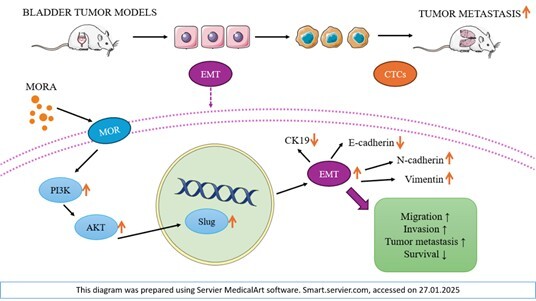
Wang X., Zhang S., Jin D., Luo J., Shi Y., Zhang Y. et al. μ‐opioid receptor agonist facilitates circulating tumor cell formation in bladder cancer via the MOR/AKT/Slug pathway: a comprehensive study including randomized controlled trial. Cancer Commun. (Lond.) 2023; 43(3): 365–386, doi: 10.1002/cac2.12408.
75.
Sessler D.I., Pei L., Huang Y., Fleischmann E., Marhofer P., Kurz A. et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019; 394(10211): 1807–1815, doi: 10.1016/S0140-6736(19)32313-X.
76.
Du Y.T., Li Y.W., Zhao B.J., Guo X.Y., Feng Y., Zuo M.Z. et al. Long-term survival after combined epidural-general anesthesia or general anesthesia alone: follow-up of a randomized trial. Anesthesiology 2021; 135(2): 233–245, doi: 10.1097/ALN.0000000000003835.
77.
Xu Z.Z., Li H.J., Li M.H., Huang S.M., Li X., Liu Q.H. et al. Epidural anesthesia–analgesia and recurrence-free survival after lung cancer surgery: a randomized trial. Anesthesiology 2021; 135(3): 419–432, doi: 10.1097/ALN.0000000000003873.
78.
Kushida A., Inada T., Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol. Immunotoxicol. 2007; 29(3–4): 477–486, doi: 10.1080/08923970701675085.
79.
Sun D., Li K., Chai Z., Wang L., Gu S., Sun N. et al. Effects of propofol intravenous general anesthesia and inhalational anesthesia on T-lymphocyte activity after breast cancer surgery: a meta-analysis. J. Res. Med. Sci. 2024; 28: 86, doi: 10.4103/jrms.jrms_336_23.
80.
Sessler D.I., Riedel B. Anesthesia and cancer recurrence: context for divergent study outcomes. Anesthesiology 2019; 130(1): 3–5, doi: 10.1097/ALN.0000000000002506.
81.
Nepogodiev D., Martin J., Biccard B., Makupe A., Bhangu A. Global burden of postoperative death. Lancet 2019; 393(10170): 401, doi: 10.1016/S0140-6736(18)33139-8.
82.
Lucas A.L., Malvezzi M., Carioli G., Negri E., La Vecchia C., Boffetta P. et al. Global trends in pancreatic cancer mortality from 1980 through 2013 and predictions for 2017. Clin. Gastroenterol. Hepatol. 2016; 14(10): 1452–1462.e4, doi: 10.1016/j.cgh.2016.05.034.
83.
Lomberk G., Dusetti N., Iovanna J., Urrutia R. Emerging epigenomic landscapes of pancreatic cancer in the era of precision medicine. Nat. Commun. 2019; 10(1): 3875, doi: 10.1038/s41467-019-11812-7.
84.
Weiser T.G., Haynes A.B., Molina G., Lipsitz S.R., Esquivel M.M., Uribe-Leitz T. et al. Size and distribution of the global volume of surgery in 2012. Bull. World Health Organ. 2016; 94(3): 201–209F, doi: 10.2471/BLT.15.159293.
85.
Souza K.M., De Vivo I., Chen C.Y., Nogueira F.R., Aun A.G., Arruda N.M. et al. Oxidative stress, DNA damage, inflammation and gene expression in occupationally exposed university hospital anesthesia providers. Environ. Mol. Mutagen. 2021; 62(2): 155–164, doi: 10.1002/em.22420.
86.
Varughese S., Ahmed R. Environmental and occupational considerations of anesthesia: a narrative review and update. Anesth. Analg. 2021; 133(4): 826–835, doi: 10.1213/ANE.0000000000005504.
87.
Turillazzi E., Bello S., Bonsignore A., Neri M., Riezzo I., Fineschi V. Retrospective analysis of anaesthesia-related deaths during a 12-year period: looking at the data from a forensic point of view. Med. Sci. Law 2012; 52(2): 112–115, doi: 10.1258/msl.2011.011074.
88.
Li G., Warner M., Lang B.H., Huang L., Sun L.S. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology 2009; 110(4): 759–765, doi: 10.1097/aln.0b013e31819b5bdc.
89.
Skulska A., Kała M., Parczewski A. Fentanyl and its analogues in clinical and forensic toxicology. Przegl. Lek. 2005; 62(6): 581–584.
CYTOWANIA (1):
1.
Immunomodulatory effects of anesthetic drugs and their implications in neurodegenerative disease progression
Mohammed Hadi Kadhim Al-Juhaishi, Nooralhuda Mohammed Hadi Al-Juhaishi, Ahmed Mohammed Hadi Al-Juhaishi
Neurophysiology
Mohammed Hadi Kadhim Al-Juhaishi, Nooralhuda Mohammed Hadi Al-Juhaishi, Ahmed Mohammed Hadi Al-Juhaishi
Neurophysiology
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.