Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Amyloidoza serca
1
Department of Cardiology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Małgorzata Niemiec
Klinika Kardiologii, Górnośląskie Centrum Medyczne im. prof. Leszka Gieca Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Ziołowa 45/47, 40-635 Katowice
Klinika Kardiologii, Górnośląskie Centrum Medyczne im. prof. Leszka Gieca Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Ziołowa 45/47, 40-635 Katowice
Ann. Acad. Med. Siles. 2024;78:146-154
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
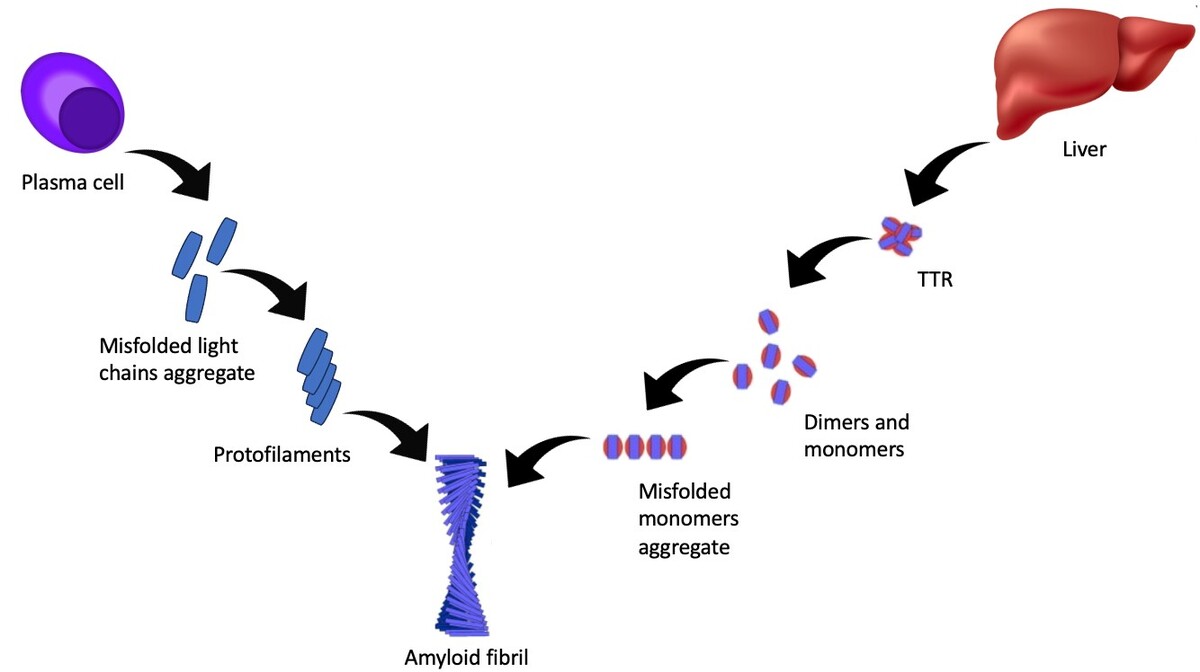
Amyloidoza jest rzadką chorobą charakteryzującą się nieprawidłowym gromadzeniem się białka amyloidowego w tkankach. Amyloidozę można podzielić na dwa główne podtypy: amyloidozę transtyretynową (ATTR-CA) i amyloidozę łańcuchów lekkich immunoglobulin (AL-CA). Nagromadzenie białka amyloidu w mięśniu sercowym może doprowadzić do zaburzeń przewodzenia, kardiomiopatii restrykcyjnej i w konsekwencji niewydolności serca. Objawy mogą obejmować spadek tolerancji wysiłku, duszność, obrzęki oraz omdlenia. Rozpoznanie opiera się na badaniach laboratoryjnych, obrazowych oraz biopsji. Leczenie koncentruje się głównie na spowolnieniu postępu choroby oraz leczeniu objawowym.
REFERENCJE (50)
1.
Benson M.D., Buxbaum J.N., Eisenberg D.S., Merlini G., Saraiva M.J.M., Sekijima Y. et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018; 25(4): 215–219, doi: 10.1080/13506129.2018.
2.
Kumar S.K., Gertz M.A., Lacy M.Q., Dingli D., Hayman S.R., Buadi F.K. et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin. Proc. 2011; 86(1): 12–18, doi: 10.4065/mcp.2010.0480.
3.
Kyle R.A., Larson D.R., Kurtin P.J., Kumar S., Cerhan J.R., Therneau T.M. et al. Incidence of AL amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin. Proc. 2019; 94(3): 465–471, doi: 10.1016/j.mayocp.2018.08.041.
4.
Pinney J.H., Whelan C.J., Petrie A., Dungu J., Banypersad S.M., Sattianayagam P. et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J. Am. Heart Assoc. 2013; 2(2): e000098, doi: 10.1161/JAHA.113.000098.
5.
Grogan M., Scott C.G., Kyle R.A., Zeldenrust S.R., Gertz M.A., Lin G. et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J. Am. Coll. Cardiol. 2016; 68(10): 1014–1020, doi: 10.1016/j.jacc.2016.06.033.
6.
Jacobson D.R., Alexander A.A., Tagoe C., Buxbaum J.N. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid 2015; 22(3): 171–174, doi: 10.3109/13506129.2015.1051219.
7.
González-López E., Gallego-Delgado M., Guzzo-Merello G., de Haro-Del Moral F.J., Cobo-Marcos M., Robles C. et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015; 36(38): 2585–2594, doi: 10.1093/eurheartj/ehv338.
8.
Castano A., Narotsky D.L., Hamid N., Khalique O.K., Morgenstern R., DeLuca A. et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017; 38(38): 2879–2887, doi: 10.1093/eurheartj/ehx350.
9.
Falk R.H., Alexander K.M., Liao R., Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J. Am. Coll. Cardiol. 2016; 68(12): 1323–1341, doi: 10.1016/j.jacc.2016.06.053.
10.
Brenner D.A., Jain M., Pimentel D.R., Wang B., Connors L.H., Skinner M. et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004; 94(8): 1008–1010, doi: 10.1161/01.RES.0000126569.75419.74.
11.
Gertz M.A., Comenzo R., Falk R.H., Fermand J.P., Hazenberg B.P., Hawkins P.N. et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005; 79(4): 319–328, doi: 10.1002/ajh.20381.
12.
Bhuiyan T., Helmke S., Patel A.R., Ruberg F.L., Packman J., Cheung K. et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin. Circ. Heart Fail. 2011; 4(2): 121–128, doi: 10.1161/CIRCHEARTFAILURE.109.910455.
13.
Longhi S., Quarta C.C., Milandri A., Lorenzini M., Gagliardi C., Manuzzi L. et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 2015; 22(3): 147–155, doi: 10.3109/13506129.2015.1028616.
14.
El-Am E.A., Dispenzieri A., Melduni R.M., Ammash N.M., White R.D., Hodge D.O. et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J. Am. Coll. Cardiol. 2019; 73(5): 589–597, doi: 10.1016/j.jacc.2018.10.079.
15.
Palma J.A., Gonzalez-Duarte A., Kaufmann H. Orthostatic hypotension in hereditary transthyretin amyloidosis: epidemiology, diagnosis and management. Clin. Auton. Res. 2019; 29(suppl 1): 33–44, doi: 10.1007/s10286-019-00623-x.
16.
Scully P.R., Treibel T.A., Fontana M., Lloyd G., Mullen M., Pugliese F. et al. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2018; 71(4): 463–464, doi: 10.1016/j.jacc.2017.11.037.
17.
Kristen A.V., Dengler T.J., Hegenbart U., Schonland S.O., Goldschmidt H., Sack F.U. et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008; 5(2): 235–240, doi: 10.1016/j.hrthm.2007.10.016.
18.
de Marneffe N., Dulgheru R., Ancion A., Moonen M., Lancellotti P. Cardiac amyloidosis: a review of the literature. Acta Cardiol. 2022; 77(8), 683–692, doi: 10.1080/00015385.2021.1992990.
19.
Ng P.L.F., Lim Y.C., Evangelista L.K.M., Wong R.C.C., Chai P., Sia C.H. et al. Utility and pitfalls of the electrocardiogram in the evaluation of cardiac amyloidosis. Ann. Noninvasive Electrocardiol. 2022; 27(4): e12967, doi: 10.1111/anec.12967.
20.
Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019; 73(22): 2872–2891, doi: 10.1016/j.jacc.2019.04.003.
21.
Suhr O.B., Anan I., Backman C., Karlsson A., Lindqvist P., Mörner S. et al. Do troponin and B-natriuretic peptide detect cardiomyopathy in transthyretin amyloidosis? J. Intern. Med. 2008; 263(3): 294–301, doi: 10.1111/j.1365-2796.2007.01888.x.
22.
Oghina S., Delbarre M.A., Poullot E., Belhadj K., Fanen P., Damy T. Les amyloses cardiaques: état des lieux en 2022. Rev. Med. Interne 2022; 43(9): 537–544, doi: 10.1016/j.revmed.2022.04.036.
23.
Holcman K., Kostkiewicz M., Podolec P., Rubiś P. Cardiac amyloidosis – state-of-the-art diagnosis and emerging therapies. [Article in Polish]. Folia Cardiol. 2019; 14(6): 616–624, doi: 10.5603/FC.2019.0115.
24.
Habib G., Bucciarelli-Ducci C., Caforio A.L.P., Cardim N., Charron P., Cosyns B. et al. Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document In collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017; 18(10): 1090–1121, doi: 10.1093/ehjci/jex034.
25.
Phelan D., Collier P., Thavendiranathan P., Popović Z.B., Hanna M., Plana J.C. et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012; 98(19): 1442–1448, doi: 10.1136/heartjnl-2012-302353.
26.
Gillmore J.D., Maurer M.S., Falk R.H., Merlini G., Damy T., Dispenzieri A. et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133(24): 2404–2412, doi: 10.1161/CIRCULATIONAHA.116.021612.
27.
Pan J.A., Kerwin M.J., Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc. Imaging 2020; 13(6): 1299–1310, doi: 10.1016/j.jcmg.2020.03.010.
28.
Patel A.R., Kramer C.M. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc. Imaging 2017; 10(10 Pt A): 1180–1193, doi: 10.1016/j.jcmg.2017.08.005.
29.
Ioannou A., Patel R.K., Razvi Y., Porcari A., Knight D., Martinez-Naharro A. et al. Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. JACC Cardiovasc. Imaging 2023; 16(4): 464–477, doi: 10.1016/j.jcmg.2022.07.008.
30.
Perugini E., Guidalotti P.L., Salvi F., Cooke R.M., Pettinato C., Riva L. et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005; 46(6): 1076–1084, doi: 10.1016/j.jacc.2005.05.073.
31.
Ardehali H., Qasim A., Cappola T., Howard D., Hruban R., Hare J.M. et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am. Heart J. 2004; 147(5): 919–923, doi: 10.1016/j.ahj.2003.09.020.
32.
Ramaekers J., Janssens J., Waumans L., Stessens L., Dupont M., Mullens W. et al. Indications and diagnostic yield of endomyocardial biopsies for unexplained cardiomyopathy, a single center experience. Acta Cardiol. 2020; 75(2): 138–146, doi: 10.1080/00015385.2018.1561597.
33.
Hänselmann A., Berliner D., Bauersachs J., Bavendiek U. Cardiac amyloidosis-interdisciplinary approach to diagnosis and therapy. Herz 2022; 47(4): 324–331, doi: 10.1007/s00059-022-05122-w.
34.
Vrana J.A., Gamez J.D., Madden B.J., Theis J.D., Bergen H.R. 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009; 114(24): 4957–4959, doi: 10.1182/blood-2009-07-230722.
35.
Stern L.K., Kittleson M.M. Updates in cardiac amyloidosis diagnosis and treatment. Curr. Oncol. Rep. 2021; 23(4): 47, doi: 10.1007/s11912-021-01028-8.
36.
Garcia-Pavia P., Rapezzi C., Adler Y., Arad M., Basso C., Brucato A. et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021; 42(16): 1554–1568, doi: 10.1093/eurheartj/ehab072.
37.
Damy T., Costes B., Hagège A.A., Donal E., Eicher J.C., Slama M. et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur. Heart J. 2016; 37(23): 1826–1834, doi: 10.1093/eurheartj/ehv583.
38.
Papa R., Lachmann H.J. Secondary, AA, amyloidosis. Rheum. Dis. Clin. North Am. 2018; 44(4): 585–603, doi: 10.1016/j.rdc.2018.06.004.
39.
Oghina S., Bougouin W., Bézard M., Kharoubi M., Komajda M., Cohen--Solal A. et al. The impact of patients with cardiac amyloidosis in HFpEF trials. JACC Heart Fail. 2021; 9(3): 169–178, doi: 10.1016/j.jchf.2020.12.005.
40.
Ihne S., Morbach C., Obici L., Palladini G., Störk S. Amyloidosis in heart failure. Curr. Heart Fail. Rep. 2019; 16(6): 285–303, doi: 10.1007/s11897-019-00446-x.
41.
Rind J., Chalfoun N., McNamara R. Cardiac amyloidosis: The great masquerader. Glob. Cardiol. Sci. Pract. 2018; 2018(2): 18, doi: 10.21542/gcsp.2018.18.
42.
Vermeer A.M.C., Janssen A., Boorsma P.C., Mannens M.M.A.M., Wilde A.A.M., Christiaans I. Transthyretin amyloidosis: a phenocopy of hypertrophic cardiomyopathy. Amyloid 2017; 24(2): 87–91, doi: 10.1080/13506129.2017.1322573.
43.
Maron B.J., Desai M.Y., Nishimura R.A., Spirito P., Rakowski H., Towbin J.A. et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022; 79(4): 372–389, doi: 10.1016/j.jacc.2021.12.002.
44.
Tanaka H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: special focus on speckle-tracking longitudinal strain. J. Echocardiogr. 2021; 19(2): 71–79, doi: 10.1007/s12574-020-00508-3.
45.
Rausch K., Scalia G.M., Sato K., Edwards N., Lam A.K., Platts D.G. et al. Left atrial strain imaging differentiates cardiac amyloidosis and hypertensive heart disease. Int. J. Cardiovasc. Imaging 2021; 37(1): 81–90, doi: 10.1007/s10554-020-01948-9.
46.
Sperry B.W., Vadalia A. Primer on the differential diagnosis and workup for transthyretin cardiac amyloidosis. Am. J. Cardiol. 2022; 185(Suppl 1): S11–S16, doi: 10.1016/j.amjcard.2022.10.052.
47.
Maurer M.S., Elliott P., Comenzo R., Semigran M., Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 2017; 135(14): 1357–1377, doi: 10.1161/CIRCULATIONAHA.116.024438.
48.
Kyle R.A., Linos A., Beard C.M., Linke R.P., Gertz M.A., O’Fallon W.M. et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992; 79(7): 1817–1822.
49.
Comenzo R.L. Out, out–making amyloid’s candle briefer. N. Engl. J. Med. 2015; 373(12): 1167–1169, doi: 10.1056/NEJMe1508746.
50.
Rubin J., Maurer M.S. Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu. Rev. Med. 2020; 71: 203–219, doi: 10.1146/annurev-med-052918-020140.
CYTOWANIA (1):
1.
Takotsubo syndrome – A review of recent reports
Wiktoria Ficoń, Maksymilian Dobosz
Annales Academiae Medicae Silesiensis
Wiktoria Ficoń, Maksymilian Dobosz
Annales Academiae Medicae Silesiensis
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.