Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Zespół Aicardiego – opis przypadku i przegląd piśmiennictwa
1
Students’ Scientific Club, Department of Pediatrics and Developmental Neurology, Upper Silesian Children’s Health Center, Medical University of Silesia, Katowice, Poland
2
Department of Pediatrics and Developmental Neurology, Upper Silesian Children’s Health Center, Medical University of Silesia, Katowice, Poland
3
Department of Imaging Diagnostics and Interventional Radiology, Upper Silesian Children’s Health Center, Medical University of Silesia, Katowice, Poland
Zaznaczeni autorzy mieli równy wkład w przygotowanie tego artykułu
Autor do korespondencji
Patrycja Ochman-Pasierbek
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Oddział Pediatrii i Neurologii Wieku Rozwojowego, Górnośląskie Centrum Zdrowia Dziecka im. św. Jana Pawła II, Samodzielny Publiczny Szpital Kliniczny Nr 6 Śląskiego Uniwersytetu Medycznego w Katowicach, ul. Medyków 16, 40-752 Katowice
Ann. Acad. Med. Siles. 2024;78:118-126
SŁOWA KLUCZOWE
lekoopornośćpadaczkazespół Aicardiegonapady zgięcioweubytki chorioretinalneagenezja ciała modzelowatego
DZIEDZINY
STRESZCZENIE
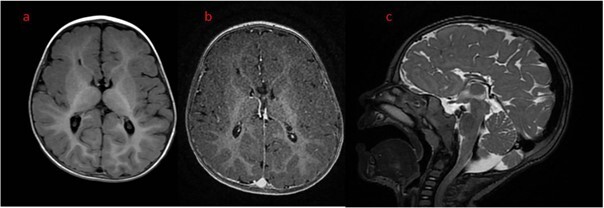
Zespół Aicardiego (Aicardi syndrome – AS) to rzadkie zaburzenie neurorozwojowe, w zdecydowanej większości przypadków występujące u dziewcząt. Zazwyczaj charakteryzuje się klasyczną triadą objawów: napadami zgięciowymi, agenezją ciała modzelowatego i chorioretinalnymi ubytkami w siatkówce. Należy również podkreślić, że padaczka lekooporna jest często głównym objawem klinicznym AS. Ponadto u pacjentów stwierdza się niepełnosprawność intelektualną, zmiany w obrębie narządu wzroku, twarzoczaszki, a także inne zaburzenia neurorozwojowe. W pracy przedstawiono przypadek pacjentki z AS z lekooporną padaczką. Pacjentka jest leczona od 3 miesiąca życia, jednak kolejne modyfikacje farmakoterapii nie doprowadziły do ustąpienia napadów. Istnieje potrzeba prowadzenia dalszych badań, aby właściwie odnieść się do kwestii skutecznego leczenia AS.
REFERENCJE (51)
1.
Aicardi J. Aicardi syndrome. Brain Dev. 2005; 27(3): 164–171, doi: 10.1016/j.braindev.2003.11.011.
2.
Shetty J., Fraser J., Goudie D., Kirkpatrick M. Aicardi syndrome in a 47 XXY male – a variable developmental phenotype? Eur. J. Paediatr. Neurol. 2014; 18(4): 529–531, doi: 10.1016/j.ejpn.2014.03.004.
3.
Wong B.K.Y., Sutton V.R. Aicardi syndrome, an unsolved mystery: Review of diagnostic features, previous attempts, and future opportunities for genetic examination. Am. J. Med. Genet. C Semin. Med. Genet. 2018; 178(4): 423–431, doi: 10.1002/ajmg.c.31658.
4.
Chappelow A.V., Reid J., Parikh S., Traboulsi E.I. Aicardi syndrome in a genotypic male. Ophthalmic Genet. 2008; 29(4): 181–183, doi: 10.1080/13816810802320209.
5.
Anderson S., Menten B., von Kogelenberg M., Robertson S., Waginger M., Mentzel H.J. et al. Aicardi syndrome in a male patient. Neuropediatrics 2009; 40(1): 39–42, doi: 10.1055/s-0029-1220760.
6.
Cuenca N.T.R., Peñaranda M.F.C., Valderrama C.A.C., Ortiz S.A., Ortiz A.F.H. Diagnostic approach to Aicardi syndrome: a case report. Radiol. Case Rep. 2022; 17(9): 3035–3039, doi: 10.1016/j.radcr.2022.05.067.
7.
Mavrommatis M.A., Friedman A.H., Fowkes M.E., Hefti M.M. Aicardi syndrome in a 20-year-old female. Am. J. Ophthalmol. Case Rep. 2018; 12: 61–64, doi: 10.1016/j.ajoc.2018.09.004.
8.
Saado S., Bara A., Abdallah Y. Aicardi syndrome in a 7-month-old girl with tonic seizures and skeletal defects: A case report. Ann. Med. Surg. 2021; 66: 102447, doi: 10.1016/j.amsu.2021.102447.
9.
Sutton V.R., Van den Veyver I.B. Aicardi Syndrome. 2006 Jun 30 [Updated 2020 Nov 12]. In: Adam M.P., Feldman J., Mirzaa G.M., et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from: https://www.ncbi.nlm.nih.gov/b... [accessed on 8 May 2024].
10.
Young R.J., Francis J.H., Abramson D.H. Aicardi syndrome. Ophthalmology 2016; 123(8): 1645, doi: 10.1016/j.ophtha.2016.04.023.
11.
Hopkins B., Sutton V.R., Lewis R.A., Van den Veyver I., Clark G. Neuroimaging aspects of Aicardi syndrome. Am. J. Med. Genet. A 2008; 146A(22): 2871–2878, doi: 10.1002/ajmg.a.32537.
12.
Shah P.K., Narendran V., Kalpana N. Aicardi syndrome: the importance of an ophthalmologist in its diagnosis. Indian J. Ophthalmol. 2009; 57(3): 234–236, doi: 10.4103/0301-4738.49403.
13.
Carney S.H., Brodsky M.C., Good W.V., Glasier C.M., Greibel M.L., Cunniff C. Aicardi syndrome: more than meets the eye. Surv. Ophthalmol. 1993; 37(6): 419–424, doi: 10.1016/0039-6257(93)90139-x.
14.
Sutton V.R., Hopkins B.J., Eble T.N., Gambhir N., Lewis R.A., Van den Veyver I.B. Facial and physical features of Aicardi syndrome: infants to teenagers. Am. J. Med. Genet. A 2005; 138A(3): 254–258, doi: 10.1002/ajmg.a.30963.
15.
Taggard D.A., Menezes A.H. Three choroid plexus papillomas in a patient with Aicardi syndrome: a case report. Pediatr. Neurosurg. 2000; 33(4): 219–223, doi: 10.1159/000055956.
16.
Pianetti Filho G., Fonseca L.F., da Silva M.C. Choroid plexus papilloma and Aicardi syndrome: case report. Arq. Neuropsiquiatr. 2002; 60(4): 1008–1010, doi: 10.1590/s0004-282x2002000600023.
17.
Krause A.C. Congenital encephalo-ophthalmic dysplasia. Arch. Ophthalmol. 1946; 36(4): 387–444, doi: 10.1001/archopht.1946.00890210395001.
18.
Fruhman G., Eble T.N., Gambhir N., Sutton V.R., Van den Veyver I.B., Lewis R.A. Ophthalmologic findings in Aicardi syndrome. J. AAPOS 2012; 16(3): 238–241, doi: 10.1016/j.jaapos.2012.01.008.
19.
Duchowny M.S., Chopra I., Niewoehner J., Wan G.J., Devine B. A systematic literature review and indirect treatment comparison of efficacy of repository corticotropin injection versus synthetic adrenocorticotropic hormone for infantile spasms. J. Health Econ. Outcomes Res. 2021; 8(1): 1–9, doi: 10.36469/jheor.2021.18727.
20.
Alrifai M.T., Al-Rumayyan A.R., Al-Tuwaijri W.A., Baarmah D.M., Asiri S.A., Bali A.H. et al. The response patterns of infantile spasms to treatments in 156 patients: Hormonal therapy with intravenous synthetic ACTH appears promising. Neurosciences (Riyadh) 2022; 27(1): 40–44, doi: 10.17712/nsj.2022.1.20210116.
21.
Riikonen R., Lähdetie J., Kokki H. ACTH treatment of infantile spasms: low-moderate- versus high-dose, natural nersus synthetic ACTH – a retrospective cohort study. Pediatr. Neurol. 2020; 111: 46–50, doi: 10.1016/j.pediatrneurol.2020.06.010.
22.
Iimura Y., Sugano H., Mitsuhashi T., Ueda T., Karagiozov K., Abe S. et al. Case report: Subtotal hemispherotomy modulates the epileptic spasms in Aicardi syndrome. Front. Neurol. 2021; 12: 683729, doi: 10.3389/fneur.2021.683729.
23.
Rosser T.L., Acosta M.T., Packer R.J. Aicardi syndrome: spectrum of disease and long-term prognosis in 77 females. Pediatr. Neurol. 2002; 27(5): 343–346, doi: 10.1016/s0887-8994(02)00450-2.
24.
Sondhi V., Agarwala A., Pandey R.M., Chakrabarty B., Jauhari P., Lodha R. et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: A randomized clinical trial. JAMA Pediatr. 2020; 174(10): 944–951, doi: 10.1001/jamapediatrics.2020.2282.
25.
Neal E.G., Chaffe H., Schwartz R.H., Lawson M.S., Edwards N., Fitzsimmons G. et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008; 7(6): 500–506, doi: 10.1016/S1474-4422(08)70092-9.
26.
Cross J.H., Neal E.G. The ketogenic diet – update on recent clinical trials. Epilepsia 2008; 49 Suppl 8: 6–10, doi: 10.1111/j.1528-1167.2008.01822.x.
27.
Podkorytova I., Gupta A., Wyllie E., Moosa A., Bingaman W., Prayson R. et al. Aicardi syndrome: epilepsy surgery as a palliative treatment option for selected patients and pathological findings. Epileptic Disord. 2016; 18(4): 431–439, doi: 10.1684/epd.2016.0872.
28.
Saito Y., Sugai K., Nakagawa E., Sakuma H., Komaki H., Sasaki M. et al. Treatment of epilepsy in severely disabled children with bilateral brain malformations. J. Neurol. Sci. 2009; 277(1–2): 37–49, doi: 10.1016/j.jns.2008.10.009.
29.
Lund C., Bjørnvold M., Tuft M., Kostov H., Røsby O., Selmer K.K. Aicardi syndrome: an epidemiologic and clinical study in Norway. Pediatr. Neurol. 2015; 52(2): 182–186.e3, doi: 10.1016/j.pediatrneurol.2014.10.022.
30.
Kasasbeh A.S., Gurnett C.A., Smyth M.D. Palliative epilepsy surgery in Aicardi syndrome: a case series and review of literature. Childs Nerv. Syst. 2014; 30(3): 497–503, doi: 10.1007/s00381-013-2259-5.
31.
Bernstock J.D., Olsen H.E., Segar D., Huang K., Kappel A.D., Essayed W. et al. Corpus callosotomy for refractory epilepsy in Aicardi syndrome: case report and focused review of the literature. World Neurosurg. 2020; 142: 450–455, doi: 10.1016/j.wneu.2020.06.230.
32.
Govil-Dalela T., Kumar A., Agarwal R., Chugani H.T. Agenesis of the corpus callosum and Aicardi syndrome: a neuroimaging and clinical comparison. Pediatr. Neurol. 2017; 68: 44–48.e2, doi: 10.1016/j.pediatrneurol.2016.12.002.
33.
Palmér L., Zetterlund B., Hard A.L., Steneryd K., Kyllerman M. Aicardi syndrome: follow-up investigation of Swedish children born in 1975–2002. Neuropediatrics 2007; 38(4): 188–192, doi: 10.1055/s-2007-991146.
34.
Wanigasinghe J., Arambepola C., Ranganathan S.S., Sumanasena S., Attanapola G. Randomized, single-blind, parallel clinical trial on efficacy of oral prednisolone versus intramuscular corticotropin on immediate and continued spasm control in West syndrome. Pediatr. Neurol. 2015; 53(3): 193–199, doi: 10.1016/j.pediatrneurol.2015.05.004.
35.
Wanigasinghe J., Arambepola C., Ranganathan S.S., Jayasundara K., Weerasinghe A., Wickramarachchi P. Epilepsy outcome at four years in a randomized clinical trial comparing oral prednisolone and intramuscular ACTH in West syndrome. Pediatr. Neurol. 2021; 119: 22–26, doi: 10.1016/j.pediatrneurol.2021.01.008.
36.
Gowda V.K., Narayanaswamy V., Shivappa S.K., Benakappa N., Benakappa A. Corticotrophin-ACTH in comparison to prednisolone in West wyndrome – a randomized study. Indian J. Pediatr. 2019; 86(2): 165–170, doi: 10.1007/s12098-018-2782-1.
37.
Chatterjee A., Mundlamuri R.C., Kenchaiah R., Asranna A., Nagappa M., Bindu P.S. et al. Role of pulse methylprednisolone in epileptic encephalopathy: A retrospective observational analysis. Epilepsy Res. 2021; 173: 106611, doi: 10.1016/j.eplepsyres.2021.106611.
38.
O’Callaghan F.J., Edwards S.W., Alber F.D., Hancock E., Johnson A.L., Kennedy C.R. et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017; 16(1): 33–42, doi: 10.1016/S1474-4422(16)30294-0.
39.
O’Callaghan F.J.K., Edwards S.W., Alber F.D., Cortina Borja M., Hancock E., Johnson A.L. et al. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolesc. Health 2018; 2(10): 715–725, doi: 10.1016/S2352-4642(18)30244-X.
40.
Eble T.N., Sutton V.R., Sangi-Haghpeykar H., Wang X., Jin W., Lewis R.A. et al. Non-random X chromosome inactivation in Aicardi syndrome. Hum. Genet. 2009; 125(2): 211–216, doi: 10.1007/s00439-008-0615-4.
41.
Schrauwen I., Szelinger S., Siniard A.L., Corneveaux J.J., Kurdoglu A., Richholt R. et al. A de novo mutation in TEAD1 causes non-X-linked Aicardi syndrome. Invest. Ophthalmol. Vis. Sci. 2015; 56(6): 3896–3904, doi: 10.1167/iovs.14-16261.
42.
Pons M.E., Garcia C.A. Aicardi syndrome in monozygotic twins. Ophthalmic Genet. 2008; 29(2): 87–88, doi: 10.1080/13816810801968669.
43.
Donnenfeld A.E., Packer R.J., Zackai E.H., Chee C.M., Sellinger B., Emanuel B.S. Clinical, cytogenetic, and pedigree findings in 18 cases of Aicardi syndrome. Am. J. Med. Genet. 1989; 32(4): 461–467, doi: 10.1002/ajmg.1320320405.
44.
Aicardi J. Aicardi syndrome: old and new findings. Int. Pediatr. 1999; 14(1): 5–8.
45.
Costa T., Greer W., Rysiecki G., Buncic J.R., Ray P.N. Monozygotic twins discordant for Aicardi syndrome. J. Med. Genet. 1997; 34(8): 688–691, doi: 10.1136/jmg.34.8.688.
46.
Wong B.K.Y., Sutton V.R., Lewis R.A., Van den Veyver I.B. Independent variant analysis of TEAD1 and OCEL1 in 38 Aicardi syndrome patients. Mol. Genet. Genomic Med. 2017; 5(2): 117–121, doi: 10.1002/mgg3.250.
47.
Kniffin C.L. Corpus callosum, agenesis of. OMIM – Online Mendelian Inheritance in Man. Published online 2018. Updated 02/27/2018. Available from: https://www.omim.org/entry/217... [accessed on 8 May 2024].
48.
National Cancer Institute. (2020). Muscular Dystrophy-Dystroglycanopathy (Congenital with Brain and Eye Anomalies) Type A, 1. Qeios. doi: 10.32388/WMDPG0.
49.
McKusick V.A. Encephalopathy, axonal, with necrotizing myopathy, cardiomyopathy, and cataracts. OMIM – Online Mendelian Inheritance in Man. Published online 1990. Available from: https://www.omim.org/entry/225... [accessed on 8 May 2024].
50.
Van den Veyver I.B. Microphthalmia with linear skin defects (MLS), Aicardi, and Goltz syndromes: are they related X-linked dominant male-lethal disorders? Cytogenet. Genome Res. 2002; 99(1–4): 289–296, doi: 10.1159/000071606.
51.
Chen M.H., Walsh C.A. FLNA Deficiency. 2002 Oct 8 [updated 2021 Sep 30]. In: Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. Available from: http://www.ncbi.nlm.nih.gov/pu... [accessed on 8 May 2024].
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.