Bieżący numer
O czasopiśmie
Rada Naukowa
Kolegium Redakcyjne
Polityka prawno-archiwizacyjna
Kodeks etyki publikacyjnej
Wydawca
Informacja o przetwarzaniu danych osobowych w ramach plików cookies oraz subskrypcji newslettera
Archiwum
Dla autorów
Dla recenzentów
Kontakt
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Polecamy
Śląski Uniwersytet Medyczny w Katowicach
Sklep Wydawnictw SUM
Biblioteka Główna SUM
Polityka prywatności
Deklaracja dostępności
Recenzenci
Recenzenci rocznika 2025
Recenzenci rocznika 2024
Recenzenci rocznika 2023
Recenzenci rocznika 2022
Recenzenci rocznika 2021
Recenzenci rocznika 2020
Recenzenci rocznika 2019
Recenzenci rocznika 2018
Recenzenci rocznika 2017
Recenzenci rocznika 2016
Recenzenci rocznika 2015
Recenzenci rocznika 2014
Recenzenci rocznika 2013
Recenzenci rocznika 2012
Stężenie chemeryny i leptyny u noworodków zdrowych i chorych
1
Department of Neonatal Intensive Care and Pathology, Faculty of Medical Sciences in Zabrze,
Medical University of Silesia, Katowice, Poland
Autor do korespondencji
Alicja Nawrat
Klinika Intensywnej Terapii i Patologii Noworodka, Wydział Nauk Medycznych w Zabrzu ŚUM, ul. 3 Maja 13, 41-800 Zabrze
Klinika Intensywnej Terapii i Patologii Noworodka, Wydział Nauk Medycznych w Zabrzu ŚUM, ul. 3 Maja 13, 41-800 Zabrze
Ann. Acad. Med. Siles. 2025;79:430-437
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wstęp:
Chemeryna i leptyna są adipokinami uczestniczącymi w regulacji metabolizmu i odpowiedzi zapalnej. W okresie noworodkowym mogą odzwierciedlać zarówno stan odżywienia płodu, jak i wczesne zaburzenia adaptacyjne związane z zakażeniem lub czynnikami ryzyka okołoporodowego.
Materiał i metody:
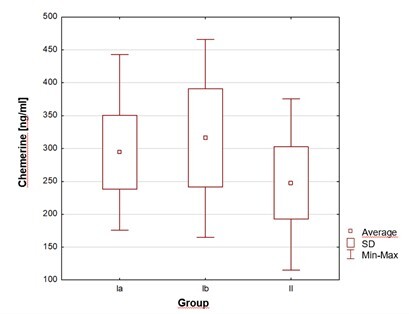
Badaniem objęto 127 noworodków donoszonych, podzielonych na grupy: Ia – z zakażeniem wczesnym (n = 40), Ib – z czynnikami ryzyka okołoporodowego (n = 36), II – kontrolną (n = 51). Stężenia chemeryny i leptyny w surowicy krwi żylnej oznaczono między 3. a 7. dobą życia. Analizowano zależności między parametrami hormonalnymi, metabolicznymi i klinicznymi.
Wyniki:
Stężenia chemeryny i leptyny były istotnie wyższe u noworodków z wczesnym zakażeniem i obciążonych perinatalnymi czynnikami ryzyka niż u zdrowych.
Wnioski:
Chemeryna i leptyna są markerami zaburzeń metabolicznych i zapalnych w okresie okołoporodowym. Oznaczenie ich stężeń w surowicy obwodowej krwi żylnej może stanowić cenne narzędzie w identyfikacji noworodków wymagających zwiększonego nadzoru klinicznego.
Chemeryna i leptyna są adipokinami uczestniczącymi w regulacji metabolizmu i odpowiedzi zapalnej. W okresie noworodkowym mogą odzwierciedlać zarówno stan odżywienia płodu, jak i wczesne zaburzenia adaptacyjne związane z zakażeniem lub czynnikami ryzyka okołoporodowego.
Materiał i metody:
Badaniem objęto 127 noworodków donoszonych, podzielonych na grupy: Ia – z zakażeniem wczesnym (n = 40), Ib – z czynnikami ryzyka okołoporodowego (n = 36), II – kontrolną (n = 51). Stężenia chemeryny i leptyny w surowicy krwi żylnej oznaczono między 3. a 7. dobą życia. Analizowano zależności między parametrami hormonalnymi, metabolicznymi i klinicznymi.
Wyniki:
Stężenia chemeryny i leptyny były istotnie wyższe u noworodków z wczesnym zakażeniem i obciążonych perinatalnymi czynnikami ryzyka niż u zdrowych.
Wnioski:
Chemeryna i leptyna są markerami zaburzeń metabolicznych i zapalnych w okresie okołoporodowym. Oznaczenie ich stężeń w surowicy obwodowej krwi żylnej może stanowić cenne narzędzie w identyfikacji noworodków wymagających zwiększonego nadzoru klinicznego.
REFERENCJE (43)
1.
Roguska J, Zubkiewicz-Kucharska A. Chemerin as an early marker of metabolic syndrome. [Article in Polish]. Pediatr Endocrinol Diabetes Metab. 2018;24(1):45–51. doi: 10.18544/PEDM-24.01.0102.
2.
Adrych K, Stojek M, Smoczynski M, Sledzinski T, Szrok-Wojtkiewicz S, Swierczynski J. Increased serum chemerin concentration in patients with chronic pancreatitis. Dig Liver Dis. 2012;44(5):393–397. doi: 10.1016/j.dld.2011.06.020.
3.
Grygiel-Górniak B, Grzelak T, Czyżewska K, Puszczewicz M. Chemerin, resistin, and adiponectin in patients with connective tissue disease. J Med Biochem. 2018;37(2):148–154. doi: 10.1515/jomb-2017-0047.
4.
Buechler C, Feder S, Haberl EM, Aslanidis C. Chemerin isoforms and activity in obesity. Int J Mol Sci. 2019;20(5):1128. doi: 10.3390/ijms200051128.
5.
Fu J, Deng HY, Hu ML, Liao LY, Li YK. Relationship between serum cystatin C, chemerin levels and subclinical atherosclerosis in type 2 diabetes mellitus patients. [Article in Chinese]. Zhonghua Yi Xue Za Zhi. 2019;99(4):307–311. doi: 10.3760/cma.j.issn.0376-2491.2019.04.014.
6.
Li C, Yan L, Song J. Plasma level of chemerin in COPD patients and the relationship between chemerin and lipid metabolism. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(7):676–683. doi: 10.11817/j.issn.1672-7347.2016.07.003.
7.
Bai F, Zheng W, Dong Y, Wang J, Garstka MA, Li R, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget. 2017;9(1):1266–1278. doi: 10.18632/oncotarget.22260.
8.
Godlewska U, Bilska B, Zegar A, Brzoza P, Borek A, Murzyn K, et al. The antimicrobial activity of chemerin-derived peptide p4 reguires oxidative conditions. J Biol Chem. 2019;294(4):1267–1278. doi: 10.1074/jbc.RA118.005495.
9.
De Palma G, Castellano G, Del Prete A, Sozzani S, Fiore N, Loverre A, et al. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011;79(11):1228–1235. doi: 10.1038/ki.2011.32.
10.
Kaneko K, Miyabe Y, Takayasu A, Fukuda S, Miyabe C, Ebisawa M, et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R158. doi: 10.1186/ar3475.
11.
Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194(6):1537–1545. doi: 10.1016/j.ajog.2005.06.064.
12.
Lazo-de-la-Vega-Monroy ML, Gonzalez-Dominguez MI, Zaina S, Sabanero M, Daza-Benítez L, Malacara JM, et al. Leptin and its receptors in human placenta of small, adequate, and large for gestational age newborns. Horm Metab Res. 2017;49(5):350–358. doi: 10.1055/s-0043-103345.
13.
Bury A, Kulik-Rechberger B, Migielska-Wołyniec M. Relationships Between Leptin Concentration and Sex, Type of Delivery and Body Weight of Newborns During First Days of Life. [Article in Polish]. Pediatr Endocrinol. 2011;10(3):29–37.
14.
Stojewska M, Szymańska A, Wiśniewska-Ulfik D, Kwiatkowska-Gruca M, Sadownik B, Mazur B, et al. Serum leptin and omentin-1 concentration in eutrophic, full-term healthy neonates. Pediatr Pol. 2014;89(5):323–328. Doi: 10.1016/j.pepo.2014.07.004.
15.
Petridou E, Mantzoros CS, Belechri M, Skalkidou A, Dessypris N, Papathoma E, et al. Neonatal leptin levels are strongly associated with female gender, birth lenght, IGF-1 levels and formula feeding. Clin Endocrinol. 2005;62(3):366–371. doi: 10.1111/j.1365-2265.2005.02225.x.
16.
Eichelmann F, Weikert C, di Giuseppe R, Biemann R, Isermann B, Schulze MB, et al. Methodological utility of chemerin as a novel biomarker of immunity and metabolism. Endocr Connect. 2017;6(5):340–347. doi: 10.1530/EC-17-0098.
17.
Zhang XY, Yang TT, Hu XF, Wen Y, Fang F, Lu HL. Circulating adipokines are associated with Kawasaki disease. Pediatr Rheumatol Online J. 2018;16(1):33. doi: 10.1186/s12969-018-0243-z.
18.
Machura E, Ziora K, Szczepańska M, Świętochowska E, Halkiewicz F, Barć-Czarnecka M, et al. Serum levels of chemerin, omentin and vaspin in children with cystic fibrosis. Pediatr Endocrinol. 2017;16:255–262. doi: 10.18544/EP-01.16.04.1679.
19.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0.
20.
Yahya RS, Awad SI, Kizilbash N, El-Baz HA, Atia G. Enteric parasites can disturb leptin and adiponectin levels in children. Arch Med Sci. 2018;14(1):101–106. doi: 10.5114/aoms.2016.60707.
21.
Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, et al. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109(1):91–95. doi: 10.1111/1523-1747.ep12276660.
22.
Jaworek J, Szklarczyk J, Kot M, Góralska M, Jaworek A, Bonior J, et al. Chemerin alleviates acute pancreatitis in the rat thorough modulation of NF-κB signal. Pancreatology. 2019;19(3):401–408. doi: 10.1016/j.pan.2019.02.005.
23.
Zhou X, Tao Y, Chen Y, Xu W, Qian Z, Lu X. Serum chemerin as a novel prognostic indicator in chronic heart failure. J Am Heart Assoc. 2019;8(15):e012091. doi: 10.1161/JAHA.119.012091.
24.
Ba HJ, Xu LL, Quin YZ, Chen HS. Serum chemerin levels correlate with determinants of metabolic syndrome in obese childrem and adolescents. Clin Med Insights Pediatr. 2019;13:1179556519853780. doi: 10.1177/1179556519853780.
25.
Weigert J, Obermeier F, Neumeier M, Wanninger J, Filarsky M, Bauer S, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm Bowel Dis. 2010;16(4):630–637. doi: 10.1002/ibd.21091.
26.
Mazaki-Tovi S, Kasher-Meron M, Hemi R, Haas J, Gat I, Lantsberg D, et al. Chemerin is present in human cord blood and is positively correlated with birthweight. Am J Obstet Gynecol. 2012;207(5):412.e1–10. doi: 10.1016/j.ajog.2012.08.008.
27.
Farias DR, Poston L, Franco-Sena AB, Moura da Silva AA, Pinto T, de Oliveira LC, et al. Maternal lipids and leptin concentrations are associated with large-for-gestational-age births: a prospective cohort study. Sci Rep. 2017;7(1):804. doi: 10.1038/s41598-017-00941y.
28.
Domali E, Messinis IE. Leptin in pregnancy. J Matern Fetal Neonatal Med. 2002;12(4):222–230. doi: 10.1080/jmf.12.4.222.230.
29.
Lepsch J, Farias DR, Vaz Jdos S, de Jesus Pereira Pinto T, da Silva Lima N, Freitas Vilela AA, et al. Serum saturated fatty acid decreases plasma adiponectin and leptin throughout pregnancy independently of BMI. Nutrition. 2016;32(7–8):740–747. doi: 10.1016/j.nut.2016.01.016.
30.
Liang Z, Zhou M, Xu XK, Qu F, Chen D. Is chemerin associated with gestational diabetes mellitus? An evidence-based clinical research from Chinese women. J Obstet Gynaecol. 2018;38(4):482–487. doi: 10.1080/01443615.2017.1385596.
31.
Reynolds LJ, Chavan NR, DeHoff LB, Preston JD, Maddox HF, O’Brien JM, et al. Smoking during pregnancy increases chemerin expression in neonatal tissue. Exp Physiol. 2019;104(1):93–99. doi: 10.1113/EP087307.
32.
Janisse JJ, Bailey BA, Ager J, Sokol RJ. Alcohol, tobacco, cocaine, and marijuana use: relative constributions to preterm delivery and fetal growth restriction. Subst Abus. 2014;35(1):60–67. doi: 10.1080/08897077.2013.804483.
33.
Lona Reyes JC, Perez Ramirez RO, Llamas Ramos L, Gómez Ruiz LM, Benítez Vázquez EA, Rodríguez Patino V. Neonatal mortality and associated factors in newborn infants admitted to a Neonatal Care Unit. Arch Argent Pediatr. 2018;116(1):42–48. doi: 10.5546/aap.2018.eng.42.
34.
Ye P, Zhao N, Shu J, Shen H, Wang Y, Chen L, et al. Laparoscopy versus open surgery for adnexal masses in pregnancy: a meta-analytic review. Arch Gynecol Obstet. 2019;299(3):625–634. doi: 10.1007/s00404-018-05039-y.
35.
Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med. 2018;46(5):465–488. doi: 10.1515/jpm-2017-0027.
36.
Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Szczygiel B, et al. Chemerin, vaspin and insulin resistance in chronic hepatitis C. J Viral Hepat. 2010;17(9):661–667. doi: 10.1111/j.1365-2893.2009.01224.x.
37.
Du XY, Leung LLK. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim Biophys Sin. 2009;41(12):973–979. doi: 10.1093/abbs/gmp091.
38.
Lavis P, Bondue B, Cardozo AK. The Dual Role of Chemerin in Lung Diseases. Cells. 2024;13(2):171. doi: 10.3390/cells13020171.
39.
Toaima NN, El-Owaidy RH, Zaki DL, Eldin LB. Infections in children with simple obesity: The relation to phagocytic function and serum leptin. J Infect Public Health. 2019;12(1):57–61. doi: 10.1016/j.jiph.2018.08.007.
40.
Korek E, Krauss H. Novel adipokines: their potential role in the pathogenesis of obesity and metabolic disorders. [Article in Polish]. Postepy Hig Med. Dosw. 2015;69:799–810.
41.
Frazer-Llado T, Reyes G, Garcia I, Jeanville P. Low expression of the obese (ob/ob) gene product leptin in critically ill infants. Pediatr Res. 1996;39(Suppl 4):309. doi: 10.1203/00006450-199604001-01863.
42.
Orbak Z, Ertekin V, Akcay F, Ozkan B, Ors R. Serum leptin levels in neonatal bacterial septicemia. J Pediatr Endocrinol Metab. 2003;16(5):727–731. doi: 10.1515/jpem.2003.16.5.727.
43.
Sadownik B, Lukas W, Behrendt J, Stojewska M, Kwiatkowska-Gruca M, Rygiel K, et al. An analysis of factors determining serum leptin concentrations in healthy and infected newborns. Neuro Endocrinol Lett. 2010;31(2):221–228.
Udostępnij
ARTYKUŁ POWIĄZANY
Śląski Uniwersytet Medyczny w Katowicach, jako Operator Serwisu annales.sum.edu.pl, przetwarza dane osobowe zbierane podczas odwiedzania Serwisu. Realizacja funkcji pozyskiwania informacji o Użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje, zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka), a także poprzez gromadzenie logów serwera www, będącego w posiadaniu Operatora Serwisu. Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług zgodnie z Polityką prywatności.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.
Możesz wyrazić zgodę na przetwarzanie danych w tych celach, odmówić zgody lub uzyskać dostęp do bardziej szczegółowych informacji.